Sie befinden sich hier
Inhalt
Regulation of cell polarity and cytoskeletal dynamics by signalling
Central problems in the development of many diseases are loss of cell polarity and mis-regulation of the cytoskeleton. We are addressing these problems with the help of the Drosophila oogenesis system.
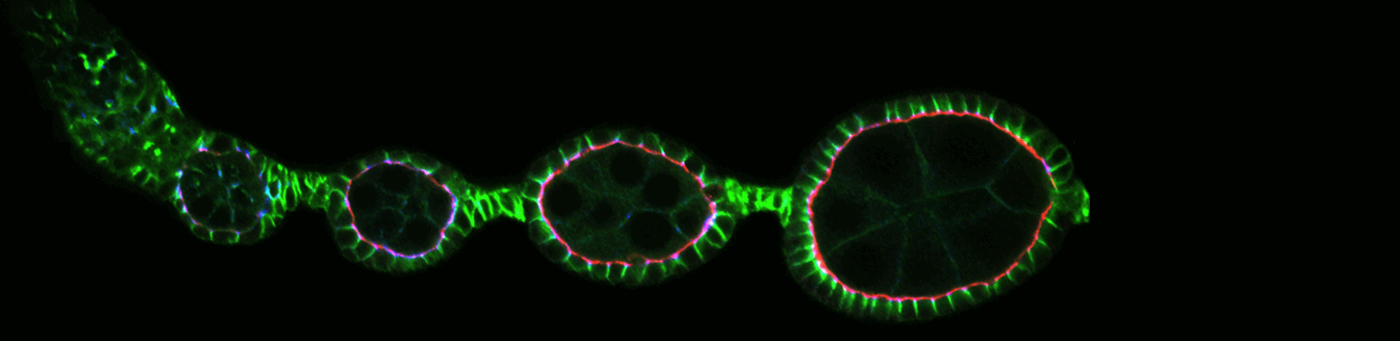
Oogenesis proceeds in simple organs consisting of a germline cyst, which is encapsulated by an epithelial layer. These egg chambers contain two cell types that are ideal to study cell polarisation and cytoskeletal dynamics in a developing organ: the oocyte and the surrounding epithelial cells.
The understanding of the mechanisms regulating polarity and cytoskeleton is relevant for several medical problems. For example, loss of polarity is a hallmark of most tumour cells. Further, the understanding of cytoskeletal regulation during morphogenesis will greatly advance our knowledge of abnormal cell behaviour in a variety of human diseases. A key question is how signalling controls cell polarisation and cytoskeletal organisation.
Our lab addresses the following questions:
How is epithelial polarity established?
The epithelial layer surrounding the germline provides a perfect model to examine how an epithelial sheet is formed and maintained in a living organism. The excellent genetic tools of the Drosophila system, a variety of markers and a brilliant histology permit a deep analysis of epithelial development. We perform genetic screens to investigate how signalling controls epithelial development.
How is cell proliferation regulated during epithelial development?
Morphogenesis of the egg chamber is controlled by an interplay between growth and cytoskeletal tension. During egg chamber development, the volume of the inner cyst increases dramatically. The epithelial surface increase, which is required to compensate this volume increase is mediated by cell divisions. Egg chambers are therefore ideal to examine how physical forces control cell proliferation. Our results suggest a mechanism, by which tension in the epithelial actin cortex triggers cell division (Wang and Riechmann, 2007). In the future, we aim to identify the signalling pathway executing this tension induced cell proliferation.
How does the oocyte actin cytoskeleton regulate axis formation?
We have shown that the actin cortex of the oocyte is composed of thick actin bundles, which anchor the microtubule cytoskeleton. Disruption of these actin bundles leads to a dramatic reorganisation of the microtubule cytoskeleton. This reorganisation prevents the establishment of the embryonic axis (Wang and Riechmann, 2008). We will further investigate how the actin cytoskeleton of the oocyte contributes to the determination of the fly body axis.
Group Members
PD Dr. Veit Riechmann
Group Leader
Dr. Nicola Berns
Postdoc
Sara Laiouar
PhD Student
Dayana Tanasic
PhD Student
Rojin Marzban
Student Assistant
Selected Publications
Berns, N., Woichansky, I., Friedrichsen, S., Kraft, N., Riechmann, V. (2014) A genome-scale in vivo RNAi analysis of epithelial development in Drosophila identifies new proliferation domains outside of the stem cell niche. Journal of Cell Science 127(12):2736-48.
Gomez, J.M., Wang, Y., Riechmann, V. (2012). Tao controls epithelial morphogenesis by promoting Fasciclin 2 endocytosis. The Journal of Cell Biology 199:1131-43. Berns, N., Woichansky, I., Kraft, N., Hüsken, U., Carl, M. and Riechmann, V. (2012) “Vacuum-assisted staining”: a simple and efficient method for screening in Drosophila. Development Genes and Evolution (in press).
JayaNandanan, N., Gavis, E.R., Riechmann, V., and Leptin, M. (2011) A genetic in vivo system to detect asymmetrically distributed RNA. EMBO Reports 12:1167-1174.
Franz, A. and Riechmann, V. (2010) Stepwise polarisation of the Drosophila follicular epithelium. Developmental Biology 338:136-147.
Wang, Y. and Riechmann, V. (2008) Microtubule anchoring by cortical actin bundles prevents streaming of the oocyte cytoplasm. Mechanisms of Development 125:142-152.
Riechmann, V. (2007) Developmental Biology: Hippo prevents posterior patterning by preventing proliferation. Current Biology 17:R1006-1008
Wang, Y. and Riechmann, V. (2007) The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Current Biology 17:1349-1355
Riechmann, V. and Ephrussi, A. (2004). Par-1 regulates bicoid mRNA localisation by phosphorylating Exuperantia. Development 131:5897-5907
Kontextspalte
Group Leader

Dr. Veit Riechmann
Phone +49 621 383-71574
veit.riechmann@medma.uni-heidelberg.de