You are here
Content
In general, our department focuses on key mechanisms driving cancer metastasis, especially micro RNAs and regulators of tumor-associated proteolysis, and their translational research potential. In this context, our interests generally include the discovery of molecular determinants defining metastasis, molecular determinants of response to novel therapeutics, molecular determinants assisting in metastasis prevention, and molecular staging of cancer.
Molecular staging models
In the past, we discovered major regulatory mechanisms of one of the most frequently overexpressed receptor molecules within tumor-associated proteolysis, the urokinase receptor (u-PAR), and suggested molecular staging models which can achieve a higher precision in the individual risk classification of patients with gastrointestinal cancers and of minimal residual disease. The group identified the EGFR-targeted therapeutic Cetuximab as an inhibitor of metastasis via blocking u-PAR expression, suggesting u-PAR as a novel biomarker for Cetuximab sensitivity. Also, the group for the first time implicated the anti-malaria agent Artesunate as a metastatis inhibitor, acting via the u-PAR ligand urokinase (u-PA) and specific matrix-metalloproteinases (MMPs).
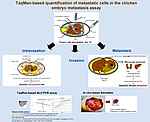
Different steps of the metastastic cascade
Furthermore, our group pioneered the novel function of tumor suppressor Pdcd4 as a metastasis suppressor by inhibiting u-PAR gene expression, and as an independent prognostic marker in colorectal cancer, and showed that Pdcd4 is essentially downregulated in cancer cells by microRNA-21.
For the recent years, our department put special emphasis on the discovery of microRNAs (miRs) and their function as key regulators of cancer metastasis. We described microRNA-21 as a key inhibitor of Pdcd4 in colorectal cancer, post-transcriptionally downregulating Pdcd4 expression and stimulating three different steps of the metastastic cascade, invasion, intravasation, and the establishment of metastatic lesions. We also implicated further microRNAs as novel regulators of metastasis of different carcinoma entities, e.g. miR-200c, miR-34a, or the miR-30-family, amongst others.
MiR-driven molecular networks
Recently, together with national and international collaborators, we extended out interest to decipher miR-driven molecular networks driving progression and metastasis and suggested a metastatically relevant network of miR-21, miR-34a, Pdcd4, Src, and PTEN. In a recent systematic profiling approach, we discovered and validated several novel miRs to be significantly deregulated in resected colorectal patient metastasis tissue as compared to the matched primary tumors and corresponding normal background tissues. From these, we functionally concluded a first novel molecular network of particular miRs which act, at least in part, via 5 novel, in part common targets, which all culminate in EMT-regulation, orchestrating invasion, in vivo intravasation and metastasis in vivo.
To contribute to identifying metastatically relevant individual cells among heterogeneous tumor cell populations, just recently our group presented the first single-cell, single molecule superresolution microscopy of human microRNAs in metastatic cancer cells in a highly interdisciplinary approach with optical biophysics. Ongoing activities in our department include the search for the molecular, genomic and epigenetic causes for miR-deregulation during the metastatic process, and the definition of factors defining site-specific metastasis based on our current data.
Systematic characterization of the genomic and molecular landscape of metastasis - the „Metastasome”
Recent activities of our department have been aiming at a more systematic characterization of the genetic and molecular landscape of human cancer metastases (the “Metastasome” as we decided to call it, see Allgayer et al., Seminars in Cancer Biology 2020). Towards this end, we have not only described the metastatically relevant microRNA landscape for colorectal cancer (Mudduluru et al., Cancer Research 2015) in translational interdisciplinary efforts, but also recently published the, at this point in time, most comprehensive whole genome sequencing study of human colorectal cancer metastases (Ishaque et al., Nature Communications 2018). In this work, we show that metastases originate from a common ancestor clone with their corresponding primary tumor, but also develop novel genomic and molecular changes that they do not share with their respective primary tumor, which led to the definition of a novel progression and metastasis model for colorectal cancer (Allgayer et al., Seminars in Cancer Biology 2020). This can have tremendous consequences for future strategies of more metastasis-specific diagnosis and personalized medicine. Therefore, our department currently, and for the future years, is putting its major research efforts on a still better molecular understanding of the “Metastasome” and of specific changes that are private to metastatic lesions, including factors that define site-specific metastasis, in increasingly interdisciplinary network activities. The ultimate aim will be to contribute to more precise, metastasis-focused therapeutic and diagnostic strategies and, ideally, metastasis prevention.
Prof. Dr. med. Heike Allgayer
Prof. Dr. med. Heike Allgayer, MD, PhD, Director and Head of the Department which is part of the Mannheim Medical Faculty of University of Heidelberg, received her MD and Dr. med. at Ludwig-Maximilians University Munich in 1995/1996, and her PhD (in Molecular Biology) at the University of Houston-MD Anderson Cancer Center in 1999. As a board-certified surgeon and molecular biologist, she sees her mission in building the bridge between basic research and metastasis prevention or treatment in cancer patients, as dedicated translational researchers (CV Prof. Allgayer). Amongst other activities and appointments, Heike Allgayer is active member of, for example, the AACR (currently serving as a member of the AACR Surgical Oncology Task Force), ASCO, the Metastasis Research Society (MRS), and EACR (serving as an Council Member and Ambassador), chair of the Fellows of the Ingrid zu Solms Foundation (IzS), and Associate Editor of the International Journal of Cancer (IJC).
Context Column
Department of Experimental Surgery - Cancer Metastasis
Medical Faculty Mannheim,
Heidelberg University
Ludolf-Krehl-Straße 13-17
68167 Mannheim
Phone +49 621 383-71630, -71635 or -71406
Fax +49 621 383-71631
experimentelle.chirurgie@medma.uni-heidelberg.de
Director and Head

Prof. Dr. med. Heike Allgayer, MD, PhD
Phone +49 621 383-71635
heike.allgayer@medma.uni-heidelberg.de