You are here
Content
Translational neurogenetics of psychiatric disorders
Our research interests focus on the development of novel treatments for severe psychiatric disorders, especially schizophrenia, through an application of multimodal neuroimaging, genetics and enviromics to characterize brain circuits underlying the risk for mental illness and cognitive dysfunction.
Many severe psychiatric disorders and complex behaviours are highly heritable. We investigate genetic variation associated with risk for disorders such as schizophrenia, depression and autism and behavioural phenomena such as attachment or impulsive violence in a translational approach, with an emphasis on multimodal neuroimaging to identify neural systems mediating genetic risk. The ultimate goal is the construction of a neural risk architecture of the studied target behaviour as guide for the discovery and evaluation of novel treatment strategies.
We are focusing on these major aspects
- Neurogenetic risk mechanisms of depression and anxiety
We study the impact on genetic risk on neural function and cognition using multimodal neuroimaging. A recent focus of that work is the investigation of epistatic interactions, relating multiple genetic variants to neural phenotypes, and the study of neural mechanisms predictive of therapeutic response and course in a longitudinal perspective. - Neural mechanisms of complex social behaviours under genetic control
Many aspects of social behaviour are highly heritable, are key components of severe psychiatric disorders that are themselves heritable, and are essential for reproductive fitness. Consequently, we have launched a program to investigate genetic contributions to the human social brain. - Genetic modulation of neural plasticity
The goal of this project is to identify the functional correlates, neurochemical mechanisms and the impact of genomic variation on experimentally induced prefrontal cortical plasticity in the human.
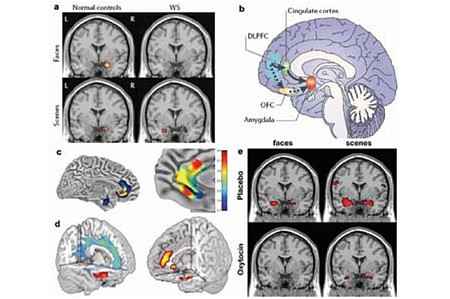
(b) Neural circuits for amygdala regulation and social function under genetic control impacted in WS (Meyer- Lindenberg et al., 2006a): both orbitofrontal cortex (OFC) and cingulate interact with amygdala in healthy controls, but OFC is disconnected in WS.
(c) structure (left) and functional connectivity (right) affected by genetic variation in 5-HTTLPR (Callicott et al., 2005). Carriers of the short allele show relative volume reductions in subgenual cingulate and amygdala, and reduced connectivity of amygdala to subgenual cingulate, delineating a feedback circuit for fear extinction and social cognition.
(d) structural (left, using voxel-based morphometry) and functional results (right, during an emotional faces matching task) show an impact of genetic variation in MAO-A on amygdala, cingulate and orbitofrontal volume and function (Meyer-Lindenberg et al., 2006c). Volume is relatively reduced in carriers of the low expression allele implicated in risk for impulsive violence. Amygdala activation is increased, while activation of regulatory cingulate and orbitofrontal regions is decreased.
(e) the prosocial hormone oxytocin modulates amygdala activation in healthy humans (Kirsch et al., 2005). Stimuli and display as in (a). Top: placebo, Bottom: oxytocin.
National and international Joint Research Projects
- BMBF: ESPRIT – Enhancing schizophrenia prevention and recovery through innovative treatments, coordinator, 02/2015-12/2021
- State Baden-Württemberg: Psychoepidemiological center (PEZ) – Psychosocial consequences of the refugee situation, 1/2017-12/2020
- Federal Innovations Fonds: MEntal Health in Refugees and Asylum Seekers, MEHIRA, 01/2017-12/2020
- BMBF: Removing language barriers in treating refugees, RELATER, 02/2019-01/2023
- BMBF: COMorbidity Modeling via Integrative Transfer machine-learning in MENTal illness (COMMITMENT), coordinator, 09/2019-08/2022
- DFG SFB 1158/2nd FP, TPB09 Regulatory brain circuits underlying bidrectional interactions between chronic pain and depression, 07/2019-06/2023
- DFG TRR265, TPS02 Mobile infrastructure for daily life research, 07/2019-06/2023
- EU AIMS-2-trials: Innovative Medicines Initiative 2, Implementation of multi-modal normative models for quantitative measures, validation and cohort stratification, 06/2018-05/2023
Selection of recent publications
- Tost, H., Champagne, F. A., & Meyer-Lindenberg, A. (2015). Environmental influence in the brain, human welfare and mental health. Nat Neurosci, 18(10), 1421-1431. doi:10.1038/nn.4108
- Tost, H., et al. Neural correlates of individual differences in affective benefit of real-life urban green space exposure. Nature neuroscience 22, 1389-1393 (2019).
- Holz, N.E., Tost, H. & Meyer-Lindenberg, A. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Molecular psychiatry (2019).
Tost H*, Reichert M, Braun U, Reinhard I, Peters R, Lautenbach S, Hoell A, Schwarz E, Ebner-Priemer U, Zipf A, Meyer-Lindenberg A*: Neural correlates of individual differences in affective benefit of real-life urban green space exposure. Nature Neuroscience, doi.org/10.1038/s41593-019-0451-y. Published online: 29 July 2019. *shared first authorship.
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A: Acute D2 receptor blockade induces rapid, reversible remodelling in human cortical-striatal circuits. Nat Neurosci, 2010 Aug;13(8):920-2.
Context Column
contact

Prof. Dr. Andreas Meyer-Lindenberg
Director and CEO)
Central Institute of Mental Health
J5
68159 Mannheim
Department of Psychiatry and Psychotherapy
Phone +49 621 1703-2002
a.meyer-lindenberg@zi-mannheim.de
