You are here
Content
Plasticity of neuronal compartments and organelles
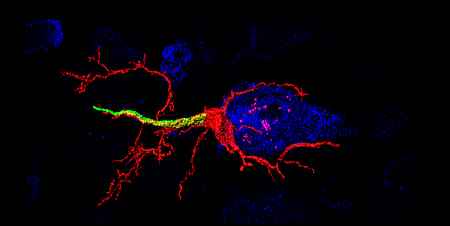
Neurons are highly polarized cells that have clearly distinguishable subcellular compartments. These compartments have characteristic structural and functional properties. The main neuronal compartments consist of the somatodendritic, axonal and synaptic domains. Our studies mainly focus on the structural plasticity of the axon. We primarily investigate the molecular composition and structural plasticity of the so-called axon initial segment (AIS).
Our main research interests are
- Axonal plasticity and development of the axon initial segment in vivo
- Neuronal polarity
- Contact between membranes of organelles
- Role of peroxisomes in neurodegeneration
The AIS corresponds to the first segment of the axon and is of crucial functional importance as the primary trigger zone for the action potential. Current research data show an amazingly strong plasticity of the AIS: Both the molecular composition and the length and position of the AIS are dynamically regulated and contribute to the regulation of neuronal excitability. In this context, we want to further elucidate the molecular basis of structural AIS plasticity - especially in the developing nervous system. Furthermore, we are investigating the mechanisms underlying the directed transport of cell organelles. This second research approach of our group aims to understand the role of membrane contacts between peroxisomes and the endoplasmic reticulum (ER) in neurons.
Recent studies show that both structure and function of AIS are dynamically regulated. One of our working hypotheses assumes that this plasticity contributes to the intrinsic excitability of neurons. Using the mouse whisker-to-barrel pathway in vivo, we have shown recently that AIS plasticity is a homeostatic, bidirectional process, which is reflected by changes in AIS length and neuronal excitability. We further uncovered two distinct timescales, in which AIS plasticity occurs in vivo. For example, the remodeling of AIS length, accompanied by significant changes in neuronal excitability, was observed within 1 hour of exposure of the animal to an enriched environment. We now aim at understanding the role of AIS plasticity within the physiological range of sensory processing and will utilize a new live reporter mouse line to begin in vivo AIS imaging studies in awake and behaving animals.
Contact sites between the membranes of peroxisomes and the endoplasmic reticulum have been shown on an ultrastructural level for quite some time. These contact sites are probably of great importance for a large number of physiological processes and the cell, including neuronal transport, intracellular lipid metabolism and the exchange of phospholipids between adjacent biomembranes. Recently, we succeeded in identifying the peroxisomal membrane protein (acyl-CoA binding protein 5; ACBD5) as a new binding partner, the ER-associated membrane protein VAPB. This was the first time that we were able to clarify the molecular basis for establishing membrane contacts between peroxisomes and ER.
In the future, the Department of Neuroanatomy will focus on the following topics:
- Development and plasticity of axon-carrying dendrites (AcD) in pyramidal neurons
- Recruitment of AcD cells into functioning hippocampal and cortical ensemble
- Untangling AIS plasticity in a reporter mouse model in vivo
- Functional relevance of membrane contacts between ER and PO
- Functional role of peroxisomes for neurodegenerative changes in the retina
Selection of recent publications
- Costello JL, Castro IG, Hacker C, Schrader TA, Metz J, Zeuschner D, Sadeghi A, Godinho LF, Costina V, Findeisen P, Manner A, Islinger M* and Schrader M* (2016) ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J. Cell Biol. in press (* shared senior authorship)
- Kubler J, Kirschner S, Hartmann L, Welzel G, Engelhardt M, Herskind C, et al. The HIV-derived protein Vpr52-96 has anti-glioma activity in vitro and in vivo. Oncotarget. 2016.
- Thome C, Kelly T, Yanez A, Schultz C, Engelhardt M, Cambridge SB, et al. Axon-carrying dendrites convey privileged synaptic input in hippocampal neurons. Neuron. 2014;83(6):1418-30.
- Schultz C, Engelhardt M. Anatomy of the hippocampal formation. Front Neurol Neurosci. 2014;34:6-17.
- Gutzmann A, Ergul N, Grossmann R, Schultz C, Wahle P, Engelhardt M. A period of structural plasticity at the axon initial segment in developing visual cortex. Front Neuroanat. 2014;8:11.
- Hirth M, Rukwied R, Gromann A, Turnquist B, Weinkauf B, Francke K, et al. Nerve growth factor induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain. 2013;154(11):2500-11.
- Engelhardt M, Vorwald S, Sobotzik JM, Bennett V, Schultz C. Ankyrin-B structurally defines terminal microdomains of peripheral somatosensory axons. Brain Struct Funct. 2013;218(4):1005-16.
- Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, et al. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci U S A. 2009;106(41):17564-9.
- Bockhart V, Constantin CE, Haussler A, Wijnvoord N, Kanngiesser M, Myrczek T, et al. Inhibitor kappaB Kinase beta deficiency in primary nociceptive neurons increases TRP channel sensitivity. J Neurosci. 2009;29(41):12919-29.
- Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9(10):1302-11.
Context Column
Contact

Prof. Dr. Christian Schultz
Neuroanatomy
Medical Faculty Mannheim
Ludolf-Krehl-Str. 13-17
68167 Mannheim
Phone +49 621 383-71555
christian.schultz@medma.uni-heidelberg.de
