You are here
Content
Visualisation of cell signaling in the neuromuscular junction: linking functions to pathology
Age-related loss of muscle mass is a feature of increasing clinical relevance in western societies. The contribution of nerve- and muscle-intrinsic changes that lead to the observed interruption of connectivity is being debated. In analogy to other ligand-gated ion channels of the central nervous system, nicotinic acetylcholine receptors (AChRs) located at the postsynaptic side of neuromuscular junctions (NMJs) display a complex activity-dependent intracellular trafficking, including exocytosis, endocytosis, recycling, and autophagic degradation.
We use a combination of sophisticated expression and imaging protocols to study AChR trafficking in live tissue and can therefore access the large regulatory capacity of nerve-muscle interaction. This revealed that activity-dependent recycling of AChR is mediated by the motor protein, myosin Va, and regulated by PKA type I. In this context, PKA function depends on proper anchoring within a NMJ-microdomain via the A-kinase anchoring protein, rapsyn. Under catabolic conditions, AChRs are degraded via autophagy employing a complex of endophilin B1, p62/SQSTM1, and the E3 ubiquitin ligase, MuRF1. This trafficking is controlled by phosphorylation of endophilin B1, which itself modulates the activity of the early endosomal organizer Rab5.
A second line of research focuses on the innervation of skeletal muscle by the sympathetic nervous system (SNS). The SNS regulates basic body functions such as heartbeat, blood pressure, and gland activities. Whereas hormone secretion from the adrenal medulla modulates these processes systemically, local and fast responses can be mediated by direct sympathetic innervation. Although many effects of the sympathetic system on skeletal muscle physiology and disease are known, direct sympathetic innervation targets in skeletal muscle have been scarcely studied. We are investigating this aspect and have recently described that NMJs are innervated by sympathetic neurons (Fig. 1A). This is of crucial importance for the integrity and function of NMJs (Fig. 1B).
We aim to further study several aspects of this innervation. These include the precise nature of interaction between sympathetic neurons and muscle fibers, the mechanisms of how sympathetic innervation modulates NMJ formation and maintenance and, finally, the links of sympathetic innervation to neuromuscular transmission disorders. The latter is particularly relevant, because sympathicomimetics have recently been successfully introduced to treat several patients suffering from congenital myasthenic syndromes, a group of inherited neuromuscular transmission disorders.
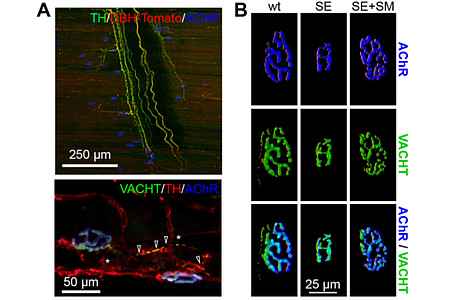
(A) Upper panel: Diaphragm muscle of a reporter mouse expressing Tomato protein (red) in sympathetic neurons was costained with anti-tyrosine hydroxylase antibody (catecholaminergic sympathetic neurons, green) and alpha-bungarotoxin (AChR, blue). Three-dimensional maximum projection of a confocal z stack of a representative region is shown. Lower panel: Longitudinal sections of wild-type EDL muscles were labeled against VACHT (cholinergic alpha motor neurons, green), tyrosine hydroxylase (catecholaminergic sympathetic neurons, red), and alpha-bungarotoxin (AChR, blue). Three-dimensional maximum projection of a confocal z stack of a representative region is shown.
(B) Sympathicomimetic treatment rescues NMJ phenotypes of sympathectomized muscle. Muscles of wild-type mice received injections of saline (wt) or 6-hydroxydopamine (sympathicotoxic, SE) on alternate days for 2 weeks. In the last 10 days, one SE group was also treated with the sympathicomimetic clenbuterol (SE+SM). Then, muscles were sectioned and stained with alpha-bungarotoxin and anti-VACHT antibody. Images show projections of representative NMJs. Figures modified from Khan et al. PNAS 2016.
Selection of recent publications
- Wild F, Khan MM, Straka T, Rudolf R. Progress of endocytic CHRN to autophagic degradation is regulated by RAB5-GTPase and T145 phosphorylation of SH3GLB1 at mouse neuromuscular junctions in vivo. Autophagy. 2016;12: 2300–2310.
- Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci U S A. 2016;113:746-50.
- Franke B, Gasch A, Rodriguez D, Chami M, Khan MM, Rudolf R, et al. Molecular basis for the fold organization and sarcomeric targeting of the muscle atrogin MuRF1. Open Biol. 2014;4:130172.
- Khan MM, Strack S, Wild F, Hanashima A, Gasch A, Brohm K, Reischl M, Carnio S, Labeit D, Sandri M, Labeit S, Rudolf R. Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy. 2014;10: 123-36.
- Choi KR, Berrera M, Reischl M, Strack S, Albrizio M, Röder IV, Wagner A, Petersen Y, Hafner M, Zaccolo M, Rudolf R. Rapsyn mediates subsynaptic anchoring of PKA type I and stabilisation of acetylcholine receptor in vivo. J Cell Sci. 2012;125: 714-723.
- Valkova C, Albrizio M, Röder IV, Schwake M, Betto R, Rudolf R, Kaether C. Rer1 controls surface-expression of muscle acetylcholine receptors by ER-retention of unassembled alpha-subunits. Proc Natl Acad Sci U S A. 2011;108: 621-625.
- Röder IV, Choi KR, Reischl M, Petersen Y, Diefenbacher ME, Zaccolo M, Pozzan T, and Rudolf R. Myosin Va cooperates with PKA RIa to mediate maintenance of the endplate in vivo. Proc Natl Acad Sci U S A 2010;107: 2031-2036.
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, and Sandri M. FoxO3 Controls Autophagy in Skeletal Muscle In Vivo. Cell Metab. 2007;6:458-471.
- Rudolf R, Magalhaes J, and Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol. 2006;173:187-193.
- Rudolf R, Mongillo M, Rizzuto R, and Pozzan T. Looking forward to seeing calcium. Nature Rev Mol Cell Biol. 2003;4:579-586.
Context Column
Contact

PD dr. Rüdiger Rudolf
Institute of Molecular and Cell Biology
Faculty of Biotechnology
Mannheim University of Applied Sciences
Paul-Wittsack-Straße 10
68163 Mannheim
Phone +49 621 292-6804
r.rudolf@hs-mannheim.de
