You are here
Content
The interaction of circulating blood cells with the vessel endothelium is a complex mechanism that is extensively explored in vascular biology research. Changes of the endothelial properties, i.e. by inflammatory processes, lead to altered interaction with blood cells and to functional activation of certain blood cells.
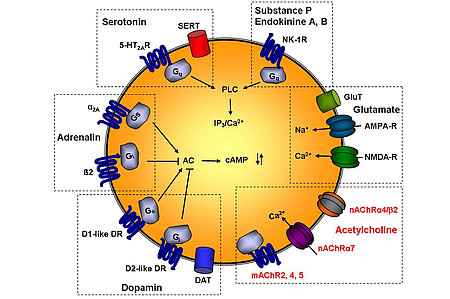
The “Platelet Biology” research group at our institute works on the identification and functional characterization of receptors involved in platelet function. Surprisingly, human platelets are equipped with a number of different receptors and transporters known from neuronal cells. The research on the functional role and clinical relevance of the neuronal receptors is one of the key aspects of our work. We were the first to show that nicotinic acetylcholine receptors are expressed on human platelets and are involved in their function.
With our anonymized DNA bank of healthy blood donors we provide an important study cohort that has been used already in numerous national and international studies for the identification of genetic risk factors for breast and other cancers, pancreatitis as well as coronary and cerebrovascular diseases.
The current research projects aim to
- investigate the expression and function of acetylcholine receptors in platelets from Alzheimer patients (in collaboration with ZI Mannheim)
- validate the platelet nAChRa7 as a peripheral blood biomarker for the Alzheimer diagnosis (in collaboration with ZI Mannheim)
- estimate the functional role and clinical relevance of the individually different expression of various platelet receptors (P2Y12, TBXA2R, nAChRa7, etc.).
The experimental work is predominantly performed on platelets from volunteer donors and patients as well as human cell culture models of megakaryopiesis. Our most important laboratory methods include
- Nucleic acid analysis (Sanger sequencing, Next-Generation-Sequencing, SNP typing, RealTime-PCR)
- Protein analysis (Western blot, flow cytometry, ELISA)
- Cell biology (humane megakaryoblastic cell lines, megakaryopoiesis from human cord blood cells, intracellular calcium imaging, cAMP measurement)
- Platelet function (impedance-aggregometry, flow cytometry, platelet-specific antibody diagnostics, adhesion measurement under flow conditions).
Project-related publications
- Yang R, Stöcker S, Schott S, Heil J, Marme F, Cuk K, Chen B, Golatta M, Zhou Y, Sutter C, Wappenschmidt B, Schmutzler R, Bugert P, Qu B, Bartram CR, Sohn C, Schneeweiss A, Burwinkel B. The association between breast cancer and S100P methylation in peripheral blood by multicenter case-control studies. Carcinogenesis 2017;38:312-320.
- Worst TS, von Hardenberg J, Gross JC, Erben P, Schnoelzer M, Hausser I, Bugert P, Michel MS, Boutros M. A database-augmented, exosome-based mass spectrometry approach exemplarily identifies circulating claudin 3 as biomarker in prostate cancer. Mol Cell Proteomics 2017 doi:10.1074/mcp.M117.068577. [Epub ahead of print]
- Tang Q, Holland-Letz T, Slynko A, Cuk K, Marme F, Schott S, Heil J, Qu B, Golatta M, Bewerunge-Hudler M, Sutter C, Surowy H, Wappenschmidt B, Schmutzler R, Hoth M, Bugert P, Bartram CR, Sohn C, Schneeweiss A, Yang R, Burwinkel B. DNA methylation array analysis identifies breast cancer associated - RPTOR, MGRN1 and RAPSN hypomethylation in peripheral blood DNA. Oncotarget 2016;7:64191-64202.
- Schedel A, Kaiser K, Uhlig S, Lorenz F, Sarin A, Starigk J, Haßmann D, Bieback K, Bugert P. Megakaryocytes and platelets express nicotinic acetylcholine receptors but nicotine does not affect megakarypoiesis or platelet function. Platelets 2016;27:43-50.
- Fjeld K, Weiss FU, Lasher D, Rosendahl J, Chen JM, Johansson BB, Kirsten H, Ruffert C, Masson E, Steine SJ, Bugert P, Cnop M, Grützmann R, Mayerle J, Mössner J, Ringdal M, Schulz HU, Sendler M, Simon P, Sztromwasser P, Torsvik J, Scholz M, Tjora E, Férec C, Witt H, Lerch MM, Njølstad PR, Johansson S, Molven A. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet 2015;47:518-522.
- Reuter B, Rodemer C, Grudzenski S, Meairs S, Bugert P, Hennerici MG, Fatar M. Effect of Simvastatin on MMPs and TIMPs in Human Brain Endothelial Cells and Experimental Stroke. Transl Stroke Res 2015;6:156-159.
- Boehringer T, Bugert P, Borggrefe M, Elmas E. SCN5A mutations and polymorphisms in patients with ventricular fibrillation during acute myocardial infarction. Mol Med Rep 2014;10:2039-2044.
- Yagmur E, Weiskirchen R, Schedel A, Bugert P. PTGS1 compound heterozygosity impairs gene expression and platelet aggregation and is associated with severe bleeding complications. Thromb Haemost 2013;110:1083-1085.
- Witt H, Beer S, Rosendahl J, Chen JM, Chandak GR, Masamune A, Bence M, Szmola R, Oracz G, Macek M, Bhatia E, Steigenberger S, Lasher D, Bühler F, Delaporte C, Tebbing J, Ludwig M, Pilsak C, Saum K, Bugert P, Masson E, Paliwal S, Bhaskar S, Sobczynska-Tomaszewska A, Bak D, Balascak I, Choudhuri G, Reddy DN, Rao GV, Varghese T, Kume K, Nakano E, Kakuta Y, Shimosegawa T, Durko L, Szabó A, Schnúr A, Hegyi P, Rakonczay Jr Z, Pfützer R, Schneider A, Groneberg DA, Braun M, Schmidt H, Witt U, Friess H, Algül H, Landt O, Schuelke M, Krüger R, Wiedenmann B, Schmidt F, Zimmer KP, Kovacs P, Stumvoll M, Blüher M, Müller T, Janecke A, Teich N, Grützmann R, Schulz HU, Mössner J, Keim V, Löhr M, Férec C, Sahin-Tóth M. Variants in CPA1 are strongly associated with early-onset chronic pancreatitis. Nat Genet 2013;45:1216-1220.
- Ait-Hsiko L, Kraaij T, Wedel J, Theisinger B, Theisinger S, Yard B, Bugert P, Schedel A. N-octanyl-dopamine is a potent inhibitor of platelet function. Platelets 2013;24:428-434.
Context Column
Contact

Peter Bugert, PhD
Associate ProfessorInstitute of Transfusion Medicine and Immunology
German Red Cross Blood Service Baden-Württemberg – Hessen gGmbH
Medical Faculty Mannheim
Heidelberg University
Friedrich-Ebert-Str. 107
68167 Mannheim
Phone +49 621 3706-8122
peter.bugert@medma.uni-heidelberg.de