Sie befinden sich hier
Inhalt
We study how the immune system drives cardiovascular and inflammatory diseases, with a particular focus on myeloid cells, innate immune sensing, and immune–vascular interactions. Our goal is to develop targeted immunopharmacological therapies that improve patient outcomes by precisely modulating key immune pathways.
Research Themes
1. Myeloid Cells in Tissue Homeostasis and Inflammation
Our research examines how macrophages and monocytes maintain tissue integrity and orchestrate immune responses. We explore the developmental origins of macrophages, distinguishing between embryonic and adult-derived populations, and investigate how tissue-resident and recruited myeloid cells differ in their functions. A particular emphasis lies on understanding how local cellular niches shape myeloid cell identity and behavior. These studies reveal how diverse myeloid populations contribute to the balance between inflammation, injury, and repair in cardiovascular and inflammatory diseases.
2. Immune–Vascular Interactions
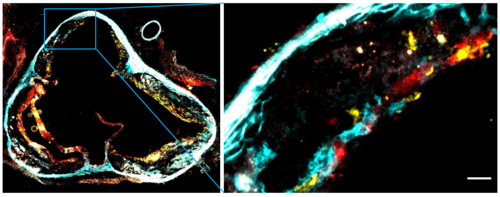
We investigate how immune mechanisms intersect with the cardiovascular system in settings such as myocardial infarction, ischemia–reperfusion injury, atherosclerosis, plaque destabilization, stroke, and thrombo-inflammatory conditions. Our work focuses on the interplay between leukocytes, endothelial cells, platelets, and vascular stromal cells, dissecting how these interactions drive vascular inflammation, remodeling, and downstream functional consequences. Through this integrated approach, we aim to identify immune pathways that critically influence cardiovascular outcomes and represent potential targets for therapeutic intervention.
3. Innate Immune Sensing and Inflammatory Signaling
A major component of our research centers on innate immune receptors and inflammasome pathways that detect tissue damage, cellular stress, and circulating danger signals. We study how sensors such as AIM2 contribute to sterile inflammation in cardiovascular disease, how innate immune responses change with age, and how they influence chronic inflammatory and metabolic disorders. By elucidating why innate immune responses become maladaptive, we aim to identify mechanisms that can be modulated to prevent excessive or harmful inflammation.
4. Immunopharmacology and Therapeutic Targeting
Our department is dedicated to translating immunological mechanisms into pharmacological strategies. We explore approaches to modulate chemokine and cytokine networks, regulate platelet-mediated immune activation, inhibit pathogenic inflammasome signaling, and enhance tissue repair after ischemic injury. We are also interested in developing age-adapted immunotherapies that address the specific challenges of immune dysfunction in older adults. This translational perspective allows us to bridge basic scientific discoveries with the development of new therapeutic approaches.
Methodological Strengths
Our laboratory employs an extensive methodological toolkit that includes genetic fate-mapping and lineage tracing of myeloid cells, intravital microscopy and advanced imaging methods to visualize immune–vascular interactions, and single-cell transcriptomic and multiomic profiling techniques. We make use of sophisticated flow cytometry platforms and imaging cytometry, combined with preclinical models of cardiovascular and inflammatory disease. Pharmacological and genetic manipulation of immune pathways, together with integration of experimental data from patient samples and clinical cohorts, enables us to develop a comprehensive understanding of immune mechanisms in complex disease settings.
Translational Vision
Our overarching vision is to harness the immune system for the development of precision therapies in cardiovascular and inflammatory diseases. We aim to identify novel therapeutic targets, establish biomarkers capable of predicting immune-driven vascular events, and improve the prevention of recurrent myocardial infarction and stroke. Moreover, we seek to mitigate excessive inflammation associated with aging and chronic disease and to contribute to the development of personalized immunopharmacological approaches for patients at high risk. Through strong collaborations with clinical partners, we strive to translate fundamental scientific insights into tangible clinical benefit.