Sie befinden sich hier
Inhalt
Our research focusses on the following topics:
- Activation of NK cells/ILCs via tumor-reactive activating receptors
- Mechanisms leading to the generation of NK memory
- NK cell subset diversification in viral infection
- Involvement of NK/ILCs in tissue damage and repair
- Clinical Immunomonitoring
Activation of Natural Killer (NK) cells/Innate Lymphoid cells (ILCs) via tumor-reactive activating receptors
Funding: DKFZ/MOST program; German Cancer Aid 70112233
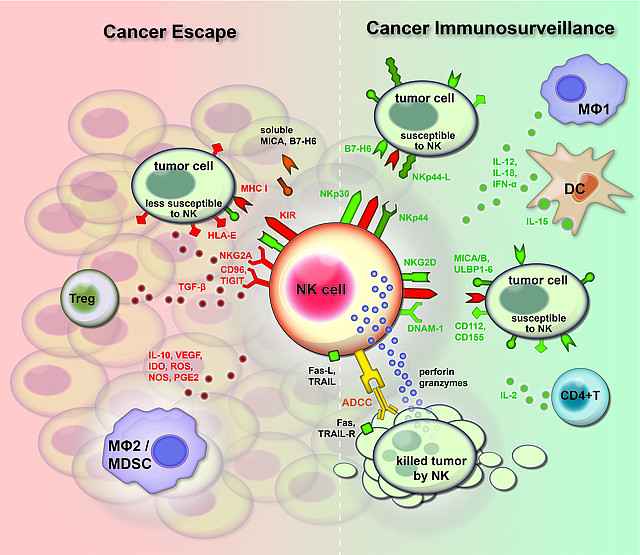
NK activation is determined by a delicate balance of activating and inhibitory receptors. Inhibitory receptors mainly bind to MHC class I on target cells. We have pioneered the field of ligands for activating receptors and identified tumor expressed ligands for the activating receptor NKG2D (Cerwenka et al, Immunity, 2000) and we have identified mechanisms of their stress induced expression on tumor cells (Textor et al., Cancer Res, 2011).
More recently, we have extended our research to Natural Cytotoxicity Receptors (NCRs) in particular to NKp30 and have produced antibodies directed to its tumor expressed ligand B7-H6. Using these novel tools, we succeeded in uncovering mechanisms of its regulation and shedding from tumors cells that might represent an important mechanism of tumor immune escape (Schlecker et al, Cancer Res, 2014; Fiegler et al, Blood, 2013). In addition, we are studying activation via another major activation receptor CD16A that is involved in the activation of therapeutic Abs.
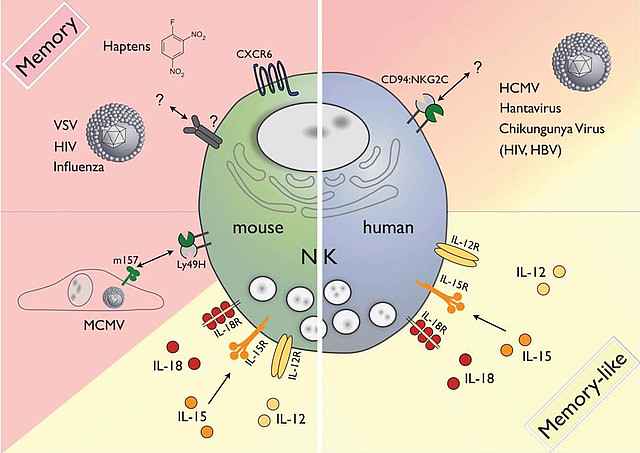
Mechanisms leading to the generation of NK cell memory
Funding: German Cancer Aid 111455; RTG 2066)
For a long time NK cells were regarded as short-lived killers. More recently, the concept has emerged that under certain conditions NK cell subsets expand and develop into memory cells (Cerwenka and Lanier, Nat. Immunol. Rev. 2016). Our studies identified IL-12 in the context of the NKG2C/HLA-E axis as a potent signal for the expansion of the NKG2C+ NK subset during human Cytomegalovirus infection (Rölle et al. JCI, 2014). Moreover, high numbers of human NK cells can be expanded in the presence of the cytokine IL-21 and feeder cells that potently inhibit the growth of melanomas in a Xenograft mouse model (Granzin et al. Oncoimmunology, 2016).
In a mouse model of lymphoma, we could generate potent anti-tumor reactive NK cells by prior exposure to the inflammatory cytokines IL-12/15/18 (Ni et al, JEM, 2012). These NK cells persisted for long-term up to 3 months in the adoptive hosts and still were able to produce high levels of the inflammatory cytokine IFN-. Their persistence of effector function coincided with epigenetic remodeling of the IFN- locus (Ni et al. Oncoimmunology, 2016). Our future studies will dissect mechanisms leading to NK memory formation and maintenance.
NK cell subset diversification in viral infection
Funding: TRR 179
Recently, NK cell expansion, contraction/exhaustion and memory responses were observed in viral infections (Rölle et al, PLOS Pathogens, 2013; Rölle et al, JCI, 2014; Pollmann et al. Front. Immunol. 2017). Based on these novel developments in the field and recent reports showing that NK cells play a critical role in control of hepatitis virus infection, in our project, we aim at obtaining novel insight into human NK cell receptors relevant in Hepatitis virus (HCV, HBV) infection and into NK cell activation/exhaustion during HCV and HBV infection. Our studies are based on in vitro immunological co-culture systems of immune cells and virus infected hepatoma cells and with HBV and HCV infected patient samples.
Involvement of NK/ILCs in tissue damage and repair
Funding: Priority Program 1937
Besides signals via inhibitory and activating receptors, NK cells in tumors are exposed to many signals from the tissue microenvironment. We have performed microarray studies and uncovered that NK cells from tumor tissue are characterized by an array of molecules of inhibitory function including CTLA-4 (Stojanovic et al. JI, 2014; Stojanovic et al, Cancer Microenviron., 2013). Moreover, we have identified transcription factors to be upregulated in tumor-infiltrated NK cells and have developed novel conditional mouse models to study their function in NK cells and other innate lymphoid cells. Ongoing research studies the response of these mice during inflammation, tissue regeneration and cancer.
Clinical Immunomonitoring
In the context of the core facility “Clinical Immunomonitoring” we are currently developing techniques for multi-parameter flow cytometry-based analysis and separation of immune subsets with a focus on innate immunity.