Sie befinden sich hier
Inhalt
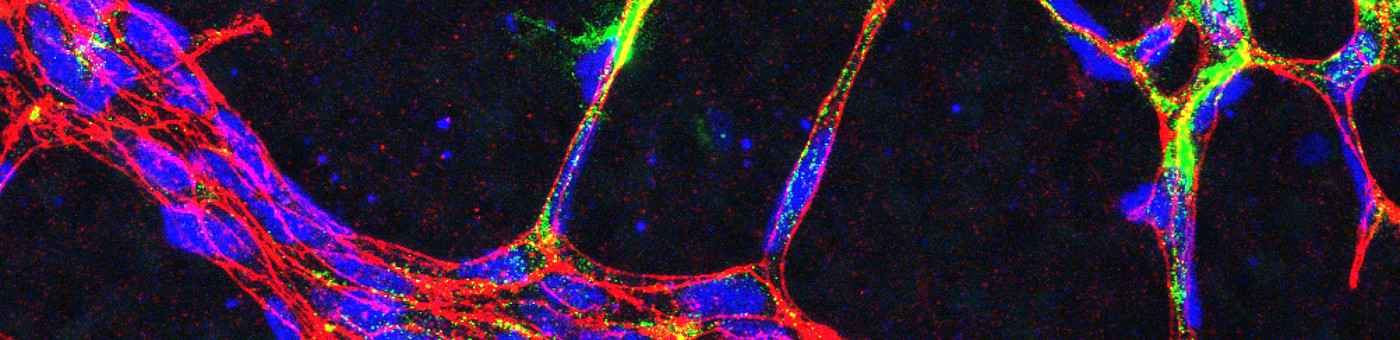
Impact of cardiac endothelial cells and fibroblasts on the progression and the development of heart failure

Although in terms of numbers endothelial cells are the most common cell type of the heart, not much is known about their contribution to the development of heart failure. In a mouse model of transverse aortic constriction, we have identified the glycoprotein CTRP9 as mainly endothelial cell derived secreted protein in the myocardium that acts in a paracrine manner to promote cardiomyocytes hypertrophy and failure by triggering a previously unknown ERK5-GATA4 signaling pathway in these cells (Appari M. et al., 2017, Circ Res).

We have also identified two novel endothelial cell derived long-non coding RNAs that promote heart failure by interfering with cardiac stress signaling (Keles M, Grein S, et al, 2024, Mol Ther Nucleic Acids). Using endothelial cell specific knock-out mouse models combined with OMICS technologies, we study central endothelial transcriptional regulators that govern the endothelial cell response to cardiac overload. (Froese N. et al., 2022, iScience; Wardman R et al, 2023, ATVB; Trogisch FA et al, 2024, Sci Transl. Med.).
Furthermore, we have recently identified the chondrocyte transcription factor SOX9 in cardiac fibroblasts as master regulator of cardiac fibrosis and inflammation, which might constitute a novel therapeutic target during myocardial infarction (Scharf G.M. et al., 2019, JCI Insight). We also found that the cardiogenic transcription factors GATA4 and GATA6 in fibroblasts prevent cardiac dysfunction by supporting myocardial angiogenesis (Dittrich GM, Froese N et al., 2021, Basic Res. Cardiol.).
Studying neonatal heart regeneration
Because adult mammals cannot sufficiently regenerate the myocardium after infarction, heart failure emerges as a long-term consequence. The dogma that mammals cannot at all regenerate the myocardium has been recently challenged by the finding that neonatal mice are able to fully regenerate their hearts after injury within the first week of life. It is now crucial to identify the molecular mechanism of full cardiac regeneration at this early stage of life and define the changes that lead to the decline of cardiac regenerative capacity after postnatal day 7.

In this regard, we found that the cardiomyocyte transcription factor GATA4 is an important inducer of neonatal cardiac regeneration, but is strongly downregulated after the first week of life (Malek Mohammadi M. et al., 2017, EMBO Mol Med and 2019, JCI Insight, 2021, Nat protocols). We are currently investigating transcriptional mechanisms of cardiac regeneration to decipher the molecular role of non-myocytes (e.g. endothelial cells) in this process.
Androgens trigger heart failure

We found that Finasteride exerts a strong anti-hypertrophic effect in mice with pressure overload, when it also improves mortality and heart function, mainly by ameliorating pro-hypertrophic signaling (Zwadlo C. et al., 2015, Circulation and Froese N. et al., 2018, JMCC). We are currently testing the efficiency of Finasteride in other cardiac diseases and are aiming to translate our findings towards the clinic (Kattih B. et al., 2019 Sci Rep).
Women have a better prognosis when suffering from heart failure or when experiencing hypertension, aortic valve stenosis or hypertrophic cardiomyopathy. One possible reason is the absence of male sex hormones, which are known to drive cardiac hypertrophy. Testosterone mainly acts via its highly active metabolite dihydrotestosterone (DHT). The generation of DHT from testosterone is driven by the enzyme 5-alpha-reductase, which can be inhibited by the anti-androgenic medication Finasteride, which is also used as therapy for benign prostate hyperplasia in men.
Healthy muscle protects the heart

Epidemiological data imply a rising influence of primarily non-cardiac disease (such as cancer, diabetes or cachexia) on heart failure. In this regard, skeletal muscle wasting is associated with a dramatically worsened prognosis in heart failure. We systematically analyze the endocrine interaction between the heart and skeletal muscle in disease models and identified several mediators of this interaction that might serve as therapeutic target in the future. We recently showed that the protective myokine Musclin becomes downregulated in skeletal muscle and in plasma of cachectic human heart failure patients as well as in mouse models of this disease. Overexpression of Musclin in skeletal muscle of mice with heart failure markedly improved cardiac dysfunction and myocardial fibrosis by enhancing the availability of protective C-type natriuretic peptide. (Szaroszyk M., Kattih B. et al., 2022, Nat Comm).
Unraveling regulatory mechanisms of cardiomyocyte protein synthesis

Pathological cardiac hypertrophy is associated with a poor prognosis in patients. It is characterized by a strong increase in cellular protein content (consisting mainly of new myofibrils) and profound changes in cardiomyocyte size and shape. We are deciphering the role of protein synthesis in the phenotype of cardiac hypertrophy and identify previously unknown negative regulators of cardiomyocyte protein synthesis, which could in the future be targeted by therapeutic approaches (Grund A. et al., 2018, Cardiovascular Research and 2019 Embo Mol Med).