Sie befinden sich hier
Inhalt
Cancer is recognized worldwide as a major health problem. Within Europe there are an estimated 3.2 million new cases and 1.3 million deaths each year. Of these deaths, it is estimated that more than 90% are due to the direct or indirect effects of metastases. Patient prognosis is therefore intimately connected with metastatic disease, as reflected in the staging systems for many types of cancer.
The diagnosis "metastatic cancer" is considered terminal for most cancer types. Metastatic disease therefore represents a major public health problem, affecting cancer patients and their families, as well as health care systems and the broader economy. Despite this, progress in developing treatments for metastatic disease remains slow.
The central aim of the Sleeman lab is to understand the process of metastasis and to use this knowledge to develop novel anti-cancer therapies.
Our Research
The interaction between tumors and the lymphatic system
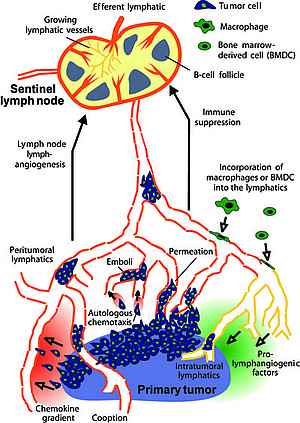
Systemic metastases arise from tumor cells that disseminate via the blood and lymphatic vasculature. Lymph nodes are often the first organs in which metastases form. They are rarely lethal of themselves, and in the main can usually be surgically removed without difficulty. However, for many types of carcinoma, the presence of metastatic tumor cells in regional lymph nodes is one of the most important indicators of poor prognosis and forms the basis of the majority of tumor staging schemes.
Sentinel lymphonodectomy, a surgical technique in which the first or "sentinel" lymph node that drains a primary tumor is removed and analysed for the presence of metastatic tumor cells, is now widely used to guide further therapy of post-operative cancer patients.
The importance of lymph node metastases in predicting the course of neoplastic disease focuses our attention on understanding how tumors interact with the lymphatic vasculature, the conduit that brings disseminating cells to regional lymph nodes, and what significance this has on determining the development of metastases in vital organs.
It is now well established that tumor-induced lymphangiogenesis is a mechanism that can promote metastasis to regional lymph nodes. It is thought to act by promoting the entry of tumor cells into the lymphatic vasculature, and /or by increasing interstitial fluid flow to the draining lymph nodes, thereby facilitating their access to and entry into the regional nodes.
The most well studied regulators of lymphangiogenesis in the tumor context are members of the VEGF/VEGFR family, particularly the archetypal regulator of lymphangiogenesis VEGFR-3 and its ligands VEGF-C and VEGF-D.
In the majority of animal models published to date, tumor-induced lymphangiogenesis induces metastasis not only to lymph nodes but also to other organs, although the mechanism behind this observation remains to be determined. Concordantly, many studies on tumor material from human cancer patients have correlated expression of pro-lymphangiogenic factors and lymphatic vessel density in and around primary tumors with lymph node metastasis formation and poor prognosis.
Projects in the lab include:
- molecular regulation of lymphangiogenesis
- local and systemic effects of pro-lymphangiogenic factors
- the role of VEGFR-3 and its ligands in the bone marrow compartment
- the development of novel inhibitors of VEGFR-3 and lymphangiogenesis
- genetic tracking of the route taken by metastatic tumor cells in vivo
- the role of lymph nodes in the metastatic dissemination of tumor cells
Genes that regulate metastasis
Much remains to be discovered about the changes in gene expression and activation of signal transduction pathways that are responsible for metastasis.
The identification of genes and pathways associated with metastasis might identify targets for the development of novel cancer therapies. To begin to address these issues, we have analyzed gene expression profiles in two rat tumor progression model systems and identified 268 genes whose expression is exclusive to or upregulated in the metastatic phenotype.
Using a number of secondary screens we have defined several genes from this panel that promote cell motility in vitro and metastasis formation in vivo. We are concentrating our efforts on the detailed functional characterization of two of these genes: Ier2 and ASAP-1.
Metastatic niches and microenvironmental regulation of metastasis
When tumor cells disseminate, the microenvironment they encounter at secondary sites determines whether the cells die, remain dormant or grow out as fulminant metastases. A microenvironment that is conducive for metastatic outgrowth has been termed the metastatic niche. Although components of such niches can be endogenously present in the microenvironment of organs where metastases develop, remodelling of the organ microenvironment to produce a metastatic niche can determine where metastases will develop. This remodelling may be induced by primary tumors through soluble factors they secrete prior to metastatic colonization of the organs (pre-metastatic niches). This can be sufficient to produce a metastatic niche capable of supporting metastatic outgrowth of disseminated tumor cells (DTCs).
However, remodelling may also be required after colonization by DTCs to produce a fully competent metastatic niche from either the endogenous miroenvironment or from a partially remodelled pre-metastatic niche. Organ-specific metastatic niche formation may underlie patterns of metastatic outgrowth. The induction of metastatic niches in the vicinity of DTCs that settled in organs years before may underlie the breakage of dormancy and subsequent metastatic outgrowth. Furthermore, the effects of chemotherapy, targeted therapy and radiotherapy on normal host tissue may ultimately serve to promote recurrence and metastasis through microenvironmental modification (therapy-induced metastasis).
Key components of the metastatic microenvironment include signalling molecules, bone marrow-derived cells and ECM remodelling. Our work aims to understand how these components are established at metastatic sites, and how they contribute to metastasis formation. An important focus of the lab is to understand the role of modified metabolism of the extracellular matrix glycosaminoglycan hyaluronan (HA) during tumor progression, in particular the effects of small HA oligosaccharides (sHA) that can accumulate in the interstitial fluid of tumors.
Projects in the lab include:
- molecular and cellular components of metastatic niches
- Influence of the immune system on the establishment of metastatic niches
- the role of the extracellular matrix in determining tumor-initiating properties in cancer cells
- the role of modified HA metabolism and the accumulation of sHA in tumor progression
Cell senescence in tumorigenesis and metastasis
The induction of cellular senescence has been proposed on one hand to act as a barrier to transformation, but on the other hand to promote tumor growth and metastasis through the senescence-associated secretory phenotype (SASP). Research in the lab focuses on the impact of senescent cells on the recruitment of pro-tumorigenic immune cells, the induction of metastasis and the response to therapy.
Control of estrogen receptor expression and function in mammary carcinoma
Approximately 70% of all mammary carcinomas are characterized by increased expression of the estrogen receptor alpha (ERα). Correct control of the expression and function of the ERα is vital to ensure that breast epithelial cells respond appropriately to estrogen. Loss of tumor suppressors that cause deregulation of ERα expression or function is considered to be one of the first and most important events that lead to the development of ER+ breast cancer. Our research aims to elucidate the molecular mechanisms that control the expression and function of ERα, and to use this knowledge to guide the development of new therapeutic approaches.