Sie befinden sich hier
Inhalt
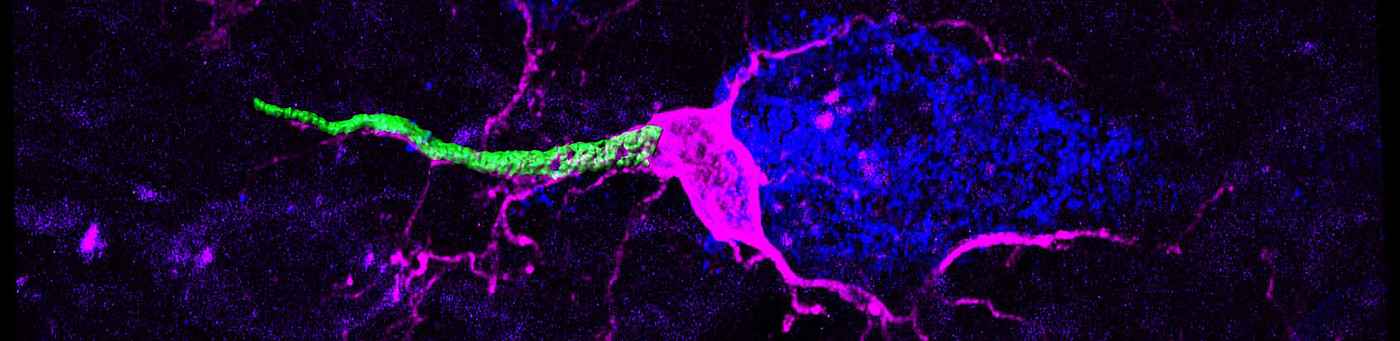
Neuronal consequences of mitochondrial dysfunction
Mitochondria are ubiquitous subcellular organelles essential for a variety of metabolic processes such as energy conversion or fatty acid oxidation as well as calcium homeostasis and apoptosis. It is thus not surprising that mitochondrial dysfunction results in complex inherited metabolic disorders particularly affecting tissues of high-energy demand, such as the nervous system. Mitochondrial diseases affect one in 5,000 individuals and display a complex genotype-phenotype relationship as well as a high degree of tissue-specificity. We are interested in understanding cellular responses to dysfunctional mitochondrial gene expression, one of the main causes of mitochondrial disease, with a special focus on the nervous system. To this end, we combine various primary cell culture models and mouse models of mitochondrial disease with recently developed first-in-class inhibitors of mitochondrial transcription (Bonekamp, Peter et al, 2020) and couple those with a variety of morphological, proteomic and biochemical analyses. We aim to get insight into the basis of the extreme variability of mitochondrial diseases, with the hope of uncovering pathways for therapeutic intervention.
Highly motivated students are always welcome!
Team Members
Lisa Wolters
PhD Student
lisa.wolters@medma.uni-heidelberg.de
Katherine Dodel
MD student
katherine.dodel@stud.uni-heidelberg.de
Alumni
Artur Astapenka, MSc
Funding
Selected publications
- Burr S.P., Klimm F., Glynos A., Prater M., Sendon P., Nash P., Powell C.A,. Simard M.L., Bonekamp N.A., Charl J., Diaz H., Bozhilova L.V., Nie Y., Zhang H., Frison M., Falkenberg M., Jones N., Minczuk M., Stewart J.B., Chinnery P.F.: Cell lineage-specific mitochondrial resilience during mammalian organogenesis. Cell. 2023 Mar 16;186(6):1212-1229.e21.
- Miranda M., Bonekamp N.A§., Kühl I.: Starting the engine of the powerhouse: mitochondrial transcription and beyond. Biol Chem. 2022 Mar 31;403(8-9):779-805. Review.
- Mennuni M., Filograna R., Felser A., Bonekamp N.A., Giavalisco P., Lytovchenko O., Larsson N.G.: Metabolic resistance to the inhibition of mitochondrial transcription revealed by CRISPR-Cas9 screen. EMBO Rep. 2022 Jan 5;23(1):e53054.
- Bonekamp N.A.§, Jiang M, Motori E., Garcia Villegas R., Koolmeister C., Atanassov I., Mesaros A., Park CB, Larsson N.G.: High levels of TFAM repress mammalian mitochondrial DNA transcription in vivo. Life Sci Alliance. 2021 Aug 30;4(11):e202101034.
- Sprenger H.-G., MacVicar T., Bahat A., Fiedler K.U., Hermans S., Ehrentraut D., Ried K., Milenkovic D., Bonekamp N.A., Larsson N.G., Nolte H., Giavalisco P., Langer T.: Cellular nucleotide imbalance triggers mitochondrial DNA-dependent innate immunity. Nat Metab. 2021 May;3(5):636-650.
- Bonekamp N.A.*, Peter B.*, Hillen H.S., Felser A., Bergbrede T., Choidas A., Horn M., Unger A., Di Lucrezia R., Atanassov I., Li X., Koch, U., Menninger S., Boros J, Habenberger P, Giavalisco P., Cramer P., Denzel M., Nussbaumer P., Klebl B., Falkenberg, M. Gustafsson C.M., Larsson N.G.: Small-molecule inhibitors of human mitochondrial DNA transcription. Nature. 2020 Dec;588(7839):712-716.
- Bonekamp N.A. and Larsson N.G.: SnapShot: Mitochondrial Nucleoid. Cell. 2018 Jan 11;172(1-2):388-388. Review.
*equal contribution
§(co-) corresponding author
Kontextspalte
Group Leader

Dr. Nina Bonekamp
Medical Faculty Mannheim
Heidelberg University
Ludolf-Krehl-Str.13-17
68167 Mannheim
Phone +49 621 383-71556
nina.bonekamp@medma.uni-heidelberg.de
https://orcid.org/0000-0001-9748-8089