Sie befinden sich hier
Inhalt
As part of the new Innovation Campus (www.health-life-sciences.de), the main research interest of my group is to investigate the regulatory mechanisms of mammalian cardiomyocyte polyploidy, with an overarching goal to identify strategies to promote cardiac repair/regeneration in adult mammals.
Cardiovascular disease is a leading cause of death worldwide. Adult mammals possess limited ability to regenerate the heart; hence, after injury (e. g., heart attack), the lost myocardium is not replaced efficiently, which significantly compromises heart function and eventually leads to heart failure. Currently, therapeutic options to cure or delay the progression of heart failure is limited, and there is a pressing need for strategies to regenerate/repair the lost myocardium.
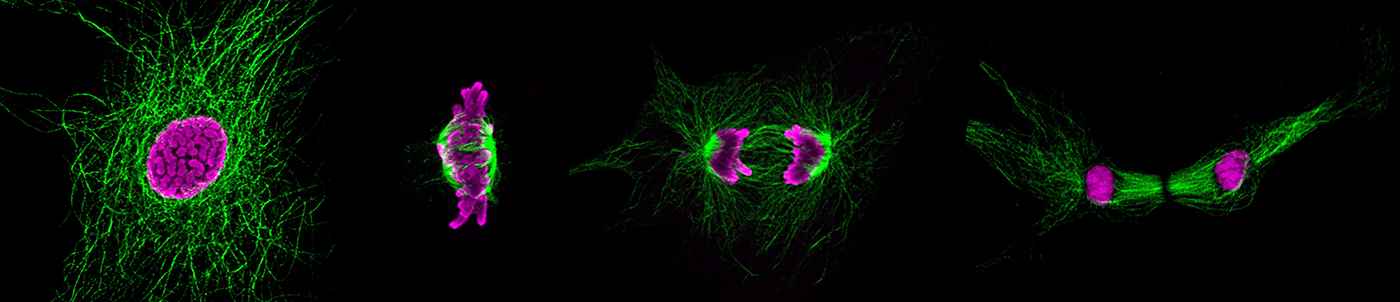
One remarkable difference between regenerative and non-regenerative organisms is cardiomyocyte ploidy. In regenerative organisms like zebrafish and newt, cardiomyocytes are primarily diploid throughout life. In contrast, mammalian cardiomyocytes become polyploid after birth, which leads to cell cycle withdrawal and the loss of endogenous regenerative capacity of the heart. Importantly, although a minor fraction of adult mammalian cardiomyocytes is able to re-enter the cell cycle after injury, they often result in increase in ploidy but not cell division, which is insufficient for heart repair. Hence, a detailed understanding of how cardiomyocyte polyploidy is regulated will help devise therapeutic strategies to promote cardiomyocyte proliferation for heart repair in mammals.
Using a variety of primary cultures, animal models, and OMIC approaches, our group aims to elucidate 1) the regulatory mechanisms and 2) the physiological significance of mammalian cardiomyocyte polyploidy. Additionally, we are also interested in understanding how polyploidy influences the function and physiology of other cell types, e. g., the endothelium.