Sie befinden sich hier
Inhalt
Topics and Principle Investigators in GRK 2727
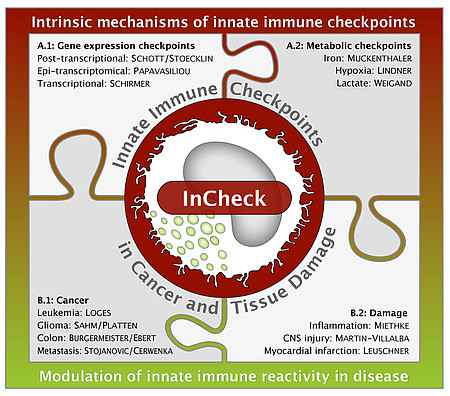
A: Cell intrinsic mechanisms of innate immune checkpoints
A.1: Gene expression checkpoints
- A.1.1: Decoding and regulating iron homeostasis in myeloid cells in neuroinflammation
- A.1.2: Epitranscriptomic control of NK cell function
- A.1.3: Translation control of checkpoint gene expression
A.2: Metabolic checkpoints
- A.2.1: Red blood cells and iron as checkpoints of macrophage polarization and NK cell reactivity
- A.2.2: Extracellular lactate as an inhibitory checkpoint of monocyte activation
- A.2.3: Understanding hypoxia-immune signaling interactions in human NK cells
B: Modulation of innate immune reactivity in disease
B.1: Cancer
- B.1.1: Modulating innate checkpoints for brain tumor immunotherapy
- B.1.2: HIF-1α-mediated regulation of liver Innate Lymphoid Cell (ILC) responses
- B.1.3: Targeting nuclear receptor checkpoints in tumor-associated macrophages from colorectal cancer
- B.1.4: Analysis of the immune checkpoint function of TAM receptors in anti-tumor NK cell response
B.2: Damage
- B.2.1: Governors of innate immunity responses after myocardial infarction: Identifying crucial healing mechanisms
- B.2.2: TIR-containing protein C of uropathogenic Escherichia coli as regulator of innate immune checkpoints
- B.2.3: CD95-Checkpoint to fine-tune innate immunity for direct efficient repair of the diseased CNS
A: Cell intrinsic mechanisms of innate immune checkpoints
A.1: Gene expression checkpoints
A.1.1:
Decoding and regulating iron homoestasis in myeloid cells in neuroinflammation
PD Dr. med. Lucas Schirmer
Multiple sclerosis (MS) is the most prevalent chronic inflammatory disease of the central nervous system leading to waves of acute inflammation with sustained immune reactions that are mainly orchestrated by innate immune cell subtypes, such as activated microglia. The inflamed lesion rim, characterized by microglia activation and macrophage recruitment, is a major pathological feature in MS. By single-nucleus RNA-sequencing (snRNA-seq), we identified a CD163- and HAMP-co-expressing disease-specific myeloid cell subtype (among other dysregulated iron pathway transcripts) that specifically mapped to the inflamed rim of chronic-active MS lesions. The aim of the project is to deconvolute and understand the role of dysregulated iron metabolism in myeloid cell subtypes and its role in initiation and progression of demyelinating neuroinflammation. In collaboration with the consortium, in particular with the laboratory of Muckenthaler, we will explore the role of activated myeloid cells in humans and in transgenic mouse models, with an emphasis on the hemoglobin-haptoglobin scavenger receptor CD163, as a novel immune checkpoint in neuroinflammation. The MD student project will focus on transcriptomic tissue mapping utilizing multiplex in situ hybridization assays.
Collaboration Partner: Dr. Simon Hametner (Medical University of Vienna)
https://www.meduniwien.ac.at/hp/phd-neuroscience/phd-students/alumni/simon-hametner/
A.1.2:
Epitranscriptomic control of NK cell function
Prof. Dr. Nina F. Papavasiliou
Natural killer (NK) cells are lymphocytes that function at the interface between innate and adaptive immunity. Cytokine secretion and direct cellular cytotoxicity are potentially dangerous responses of NK cells and therefore must be tightly controlled. NK cell reactivity is controlled by a balance of activating and inhibitory signals, delivered by the receptors which are germline-encoded and therefore invariant. Here, we hypothesize that such receptors, together with their co-receptors and the signaling pathway they specify, could be diversified at the epitranscriptomic level. NK cells express high levels of both ADAR1 and APOBEC1 editases, suggesting that they likely undergo extensive RNA modification by deamination. In preliminary experiments with resting mouse NK cells, we have found robust ADAR-editing in NK cells, which affected specific signaling pathways, including signal transduction downstream of the activating receptor NKG2D. Here, we aim to assess the functional relevance of RNA editing not only for NKG2D activity, but also for other activating NK and cytokines receptors.
A.1.3:
Translation control of checkpoint gene expression
Dr. rer. nat. Johanna Schott, Prof. Dr. med. Georg Stoecklin
When monocytes or macrophages are exposed to pathogen-associated molecular patterns, such as lipopolysaccharide (LPS), they display a rapid pro-inflammatory response followed by an anti-inflammatory phase, during which a second encounter with LPS will give rise to a strongly dampened response. In addition to transcriptional regulation, the control of mRNA stability and translation is essential for the proper temporal expression of many inflammatory genes. The Schott/Stoecklin group found a set of translationally regulated mRNAs encoding inhibitors of macrophage activation, and pursues translational regulation as an essential component of the suppressive macrophage checkpoint. Here, we propose to identify the RNA-binding protein (RBP) responsible for translational regulation of the NFκB inhibitor Nfkbid, and explore the landscape of RBPs during macrophage activation.
A.2: Metabolic checkpoints
A.2.1:
Red blood cells and iron as checkpoints of macrophage polarization and NK cell reactivity
Prof. Dr. phil. nat. Martina Muckenthaler
The overall project aim is to investigate mechanisms of how the presence of red blood cells (RBCs), heme and iron act as checkpoints for immune effector functions of macrophages and NK cells. In the tumor microenvironment, vascularization causes extravasation of RBCs and thus exposure of tumor-associated macrophages (TAMs) to heme and iron. Consequently, TAMs are polarized to a pro-inflammatory phenotype and exert direct anti-tumor effects. The overall aim is to decipher the molecular mechanism of how red blood cells, heme and iron act as checkpoints for immune effector functions of macrophages. We will apply bulk RNA sequencing, pharmacological and RNAi approaches in iron-loaded TAMs (iTAMs) to probe signaling pathways downstream of Toll-like receptors that recognize heme as a pattern, and other pathways, such as JAK/STAT signaling. In addition, we will apply an unbiased approach and perform single-cell RNA sequencing (scRNAseq) of iTAMs isolated from the tumour microenvironment of murine non-small-cell lung carcinoma (NSCLC) to understand whether these are homogenous cell populations or whether they consist of various subtypes, and how they differ from other TAMs. Combined analyses of scRNAseq data and bulk RNA sequencing will identify novel pathways that trigger the switch from the pro-tumoral to the anti-tumoral phenotype in iTAMs. The proposed studies will establish how heme and iron contribute to the functional diversity of macrophages with high relevance for anti-cancer strategies.
A.2.2:
Extracellular lactate as an inhibitory checkpoint of monocyte activation
Prof. Dr. med. Markus Weigand
In (systemic) inflammatory conditions, circulating monocytes are central drivers of the disease. Upon activation, they undergo epigenetic remodeling and metabolic reprogramming. The latter is marked by a shutdown of mitochondrial respiration, despite sufficient oxygen tension, and simultaneous upregulation of glycolysis leading to an increased lactate production. This occurs not only in the early phase of these diseases, but persists well beyond the acute phase and contributes to innate immune memory. Elevated blood lactate levels are found frequently in critically ill patients and are considered to result from tissue hypoperfusion and cellular oxygen shortage. Incorporating both aspects, we hypothesize that lactate acts as an active metabolite in inflammatory diseases rather than being a glycolytic waste product. This idea is consistent with findings in oncology, where tumor-derived lactate shapes the immune response within the tumor microenvironment. Apart from multiple effects that can be attributed to acidification due to the addition of unbuffered lactic acid, the extracellular application of buffered lactate at physiological pH dampens the pro-inflammatory cytokine response of monocytes.
The aim of our project is to unravel the mechanisms by which extracellular lactate acts as an inhibitory checkpoint that regulates monocyte function, deciphering the molecular mechanisms (both in vitro and in vivo) of monocytic adaption to extracellular lactate that leads to an attenuated inflammatory response. We will close the mechanistic gap between lactate-triggered impairment of glycolysis and the observed altered inflammatory reaction. Additionally, as a first step towards translation, the action of lactate therapy on systemic inflammation in mice will be evaluated. The MD project will assess the therapeutic potential of lactate infusion therapy to counteract hemodynamic instability in a porcine model.
A.2.3:
Understanding hypoxia-immune signaling interactions in human NK cells
PD Dr. rer. physiol. Holger Lindner
Immune cell activation is typically associated with aerobic glycolysis, which delivers amino acid precursors for effector protein biosynthesis. In myeloid cells, this is driven by hypoxia inducible factor 1α (HIF-1α). Knock-out of HIF-1α specifically in mouse T cells and NK cells, however, affects antimicrobial and antitumor activity, respectively, and thus represents an immune checkpoint. NK cells rapidly migrate to sites of inflammation where they experience hypoxia (Eltzschig & Carmeliet, N Engl J Med, 2011). We have shown that in human NK cells, short-term hypoxia applied in vitro neither affected target cell killing nor IL-12/18-induced release of chemokines / IFNγ, and that interleukin 15 (IL-15) priming enhanced the hypoxia-induced transcriptional HIF-1α pathway response. Failure to resolve inflammatory hypoxia eventually suppresses NK effector functions metabolically. The significance of HIF-1α, however, for the dynamics of metabolic and functional adaption of NK cells to hypoxia, and their phenotypic polarization - pro- versus anti-inflammatory - over the course of inflammation, remains poorly understood. Here, we hypothesize that continued hypoxic immune stimulation supports an anti-inflammatory NK cell phenotype. Modulation of the signaling network that controls HIF-1α may thus have therapeutic potential in inflammatory diseases, such as cancer and sepsis. In the PhD project, we aim at a detailed understanding of the HIF-1α regulatory network in IL-15-primed NK cells, using our recent systems immunology approach. Model predictions on the importance of different signaling modules, including STAT3, will guide the experimental characterization of interactions between immune stimulation and hypoxia through readouts of pro- and anti-inflammatory NK effector functions in vitro and in murine sepsis models. The MD project will investigate whether NK cell dysfunction seen in sepsis (Guo et al., Immunology, 2017) is associated with activation of the HIF-1α signaling network, i.e. immune checkpoint activation, and/or with emergency lymphopoiesis. Overall, we expect our results to be highly relevant for NK cell-based therapeutic strategies in cancer and sepsis.
B: Modulation of innate immune reactivity in disease
B.1: Cancer
B.1.1:
Modulating innate checkpoints for brain tumor immunotherapy
Dr. med. Katharina Sahm, Prof. Dr. med. Michael Platten
In malignant gliomas, therapy response is significantly limited by tumor-associated macrophages (TAMs), including brain-resident microglia and monocyte-derived macrophages, which account for up to 50 % of the brain tumor mass. We recently showed that innate treatment resistance could be partially overcome by local irradiation. Thus, we hypothesize that TAM modulation under the conditions induced by irradiation provides an attractive target for optimization of immunotherapy. Within this project, a PhD student will apply genetically modified syngeneic murine glioma models to identify and therapeutically modulate innate checkpoints within the brain tumor microenvironment. Specific aims are to validate the role of myeloid CXCL10 chemokine release for T cell-based glioma immunotherapy and to characterize the innate compartment in gliomas during and after radioimmunotherapy. The MD student will validate previously identified innate cell surface checkpoints in human glioma specimen.
B.1.2:
HIF-1α-mediated regulation of liver Innate Lymphoid Cell (ILC) responses
Dr. rer. nat. Ana Stojanovic, Prof. Dr. rer. nat. Adelheid Cerwenka
Cellular growth, survival and function are shaped by microenvironmental conditions, such as availability of nutrients, energy and oxygen. The transcription factor Hypoxia Inducible Factor 1 alpha (HIF-1α) is a central regulator of cellular adaptation to its microenvironment and bioenergetic demands. Factors that stabilize HIF-1α protein (hypoxia, reactive oxygen species or metabolites, such as succinate and fumarate) are common features of solid tumors, and associate with metastasis, resistance to therapy and poor patient survival (Rankin and Giaccia, Science 2016). Oxygen-deprived tissue areas and a unique metabolic environment also characterize certain organs, such as the liver. The liver tissue is enriched in innate lymphocytes, including natural killer (NK) cells and tissue-resident innate lymphoid cells (ILCs), which are early responders to tissue damage and neoplastic transformation. We recently demonstrated that conditional deletion of HIF-1α in NK cells resulted in reduced growth of subcutaneously transplanted lymphomas. In this project, we hypothesize that HIF-1α regulates innate lymphocytes in the liver organ-specific niche, both in homeostasis and in response to metastatic tumor growth. We aim to define HIF-1α-driven adaptation of innate lymphocytes to the liver microenvironment, and metabolic and microenvironmetal requirements for their optimal anti-metastatic responses in liver.
B.1.3:
Targeting nuclear receptor checkpoints in tumor-associated macrophages from colorectal cancer
PD Dr. rer. nat. Elke Burgermeister, Prof. Dr. med. Matthias Ebert
Colorectal cancers (CRCs) are largely refractory to immunotherapy. Efficacy of antibodies against cell surface checkpoints (PD-1/PD-L1) is limited to few patients (<14 %) with micro-satellite instable tumors. Macrophages (Mac) are promising effector populations for tumor immunosurveillance and elimination, which are targetable by metabolic and intracellular checkpoints beyond receptor-ligand systems at the cell surface. We found that the iron/heme-regulated transcription factor REVERBA (NR1D1) and its target gene BMAL1 (ARNTL) induced vascular-endothelial-growth-factor-A (VEGFA) expression to confer resistance to anti-angiogenic therapy in CRC. We therefore hypothesize that this drugable nuclear receptor can be exploited to reprogram Mac to alleviate their anergy in tumors and kill cancer cells. In the present proposal, we will 1. Dissect the molecular mechanisms of REVERBA-mediated regulation of Mac anergy; 2. Investigate the bi-directional cross-talk of Mac with patient-derived tumor organoids (PDOs); 3. Define the impact of clinically drugable checkpoints on Mac activation in human CRC tissues and sera. The MD project will elucidate the molecular function and therapeutic potential of the REVERBA checkpoint in NK cell in co-cultures with PDOs (collaboration Stojanovic/Cerwenka).
B.1.4:
Analysis of the immune checkpoint function of TAM receptors in anti-tumor NK cell responses
Prof. Dr. med. Dr. rer. nat. Sonja Loges
The introduction of immune checkpoint inhibitors (ICIs), which block tumor-elicited inhibitory signals on CD8+ cytotoxic T cells, has revolutionized treatment of cancer patients. However, only approximately 20 % of patients show long-term benefits. Therefore, additional strategies are needed to improve the overall efficacy of cancer immune therapy. In this context, NK cells are of special interest because they can directly kill cancer cells and recognize MHC class Ineg cells, which cannot be attacked by T cells. Previous data showed that the tumor-associated macrophage (TAM) receptors TYRO3, AXL and MER were expressed by NK cells. They function as negative immune checkpoints because their simultaneous inhibition can lead to improved tumor cell killing by NK cells. However, this approach might not feasible in humans as triple-TAM KO mice develop lethal autoimmune diseases. We hypothesize that targeting one TAM-receptor (TAMR) is sufficient to enhance the anti-tumor effector functions of NK cells, and propose to dissect the unknown functions of each individual TAMR in vitro and in vivo. We will genetically and pharmacologically block TAMR function and analyze the effect on anti-tumor NK cell responses. Based on these data, we will design preclinical trials of TAMR inhibition in combination with ICIs. The MD student will investigate the translational relevance of this approach by determining TAMR expression by circulating and tumor-infiltrating NK cells in cancer patients. From this project, we will derive strategies that will form the fundament for clinical trials in the future.
B.2: Damage
B.2.1:
Governors of innate immunity responses after myocardial infarction: Identifying crucial healing mechanisms
Prof. Dr. med. Florian Leuschner
The healing process after myocardial infarction (MI) is characterized by a biphasic, tightly orchestrated myeloid cell response. While neutrophils and pro-inflammatory monocytes (Mo) dominate the first days after MI, reparative macrophages (Mac) ensure resolution of inflammation at later stages. We hypothesize that other less abundant leukocyte subpopulations accumulating infracted tissue critically impact this switch from a pro- to an anti-inflammatory cardiac microenvironment. Single-cell RNA sequencing analyses from hearts after MI in mice led to the conclusion that basophils controled Mo/Mac polarization in the infarcted heart via Interleukin (IL)-4 and IL-13. We now aim to 1. Elucidate the effect of augmented basophil accumulation after MI and in the context of basophilia-inducing co-morbidities; and 2. Explore alterations in the translatome of Mo/Mac in the presence and absence of basophils. The MD project will investigate the role of the CTLA-4, expressed by T cells and possibly NK cells, in regulating neighboring myeloid cells after MI. Further collaborative efforts within the framework of GRK2727 will include elucidation of an involvement of other accumulating leukocytes (e.g. NK/ILCs), novel mechanisms of cardiac Mac polarization and the impact of sepsis on resident cardiac Mac populations. Advancing our understanding of healing mechanisms after MI will enhance future efforts to ameliorate and prevent ischemic heart failure.
B.2.2:
TIR-containing protein C of uropathogenic Escherichia coli as regulator of innate immune checkpoints
Prof. Dr. med. Thomas Miethke
Bacterial pathogens suppress innate immunity by expressing virulence factors, which inhibit host cell death, suppress inflammatory signaling or impair cell-intrinsic anti-microbial immunity. We discovered the TIR-domain-containing protein C (TcpC), which belongs to a new class of bacterial virulence factors. These virulence factors modulate the activation state of innate immune cells by molecular mimicry of the Toll-Interleukin-1 receptor (TIR) domain and interact with critical innate immune checkpoints, i.e. pathogen recognition receptors (PRRs) and their signaling cascade. A number of bacterial pathogens use this strategy to impair innate immunity. TcpC of the uropathogenic E. coli strain CFT073 binds to Toll-like receptor (TLR) 4 and myeloid differentiation factor 88 (MyD88), crucial components of the TLR signaling cascade, as well as to NACHT leucin-rich repeat PYD protein 3 (NLRP3) and caspase-1, two important constituents of the NLRP3 inflammasome. By modulating these checkpoints, TcpC controls cytokine secretion of innate immune cells. Kidney abscesses upon CFT073 urinary tract infection in mice are dependent on TcpC, and up to 40 % of E. coli isolates from patients with pyelonephritis contain the tcpC-gene, underlining its clinical importance. We therefore analyzed the effect of TcpC on cytokine release by the human monocytic THP-1 cell line. We observed that TcpC increased the release of IL-1β by monocytic THP-1 cells, but not in PMA-differentiated THP-1 macrophages. We thus hypothesize that the checkpoint activity of TcpC depends on the maturation state of myeloid cells through modulation of TLR- and NLRP3-signaling cascades by TcpC. We will explore the role of these signaling pathways in controlling myeloid cell maturation by TcpC. The MD student will study the TcpC effects on innate immune cells in vivo using a murine urinary tract infection (UTI) model.
B.2.3:
CD95-Checkpoint to fine-tune innate immunity for direct efficient repair of the diseased CNS
Prof. Dr. med. Ana Martin-Villalba
The innate immune system is instrumental for establishing functional neuronal ensembles in the developing, adult and diseased central nervous system (CNS). The Martin-Villalba lab has previously shown that CD95/CD95Ligand on myeloid cells is used to coordinate neurovascular branching in the embryonic CNS, and to control activation of the innate inflammatory response following CNS injuries. In order to study injury-induced activation of Neural Stem Cells (NSCs), animals will be subjected to a transient global cerebral ischemia. To this end, the bilateral common carotid artery occlusion (BCCAO: ischemia) model will be used. A 22 minutes occlusion of both common carotid arteries induces consistent neuronal damage in the striatum and the CA1 region of the hippocampus. Laser Doppler flowmetry during operation will be used to assure a drop of <20 % of baseline levels in the regional cerebral blood flow during occlusion. This is the model of choice because it is clinically relevant, as it mimics the scenario of brain damage occurring in humans after cardiac arrest. Modulation of CD95 on myeloid cells strongly influences repair of the injured CNS, e. g. after spinal cord injury, by changing the extent and quality of myeloid infiltrates. However, these qualitative changes are not understood fundamentally. We hypothesize that blockade of CD95 will influence the ratio of Ly6Chigh - to Ly6Clow cells and, thereby, increase the regenerative capacity. First step is characterizing the BCCAO ischemia model in regard of the infiltrating immune cells, especially the fractions of Ly6Chigh and Ly6Clow monocytes by flow cytometry and immunohistology in wildtype (WT) and myeloid CD95-deficient mutant mice. In a second step, single-cell transcriptomics will be used to address how CD95 shapes the innate immune response following ischemia in WT and myeloid CD95-deficient mutant mice. Finally, by blocking CD95 with a therapeutic agent, we would like to show the impact of this approach on the regenerative capacity of the brain.
Kontextspalte
GRK 2727/1 – InCheck
„Innate Immune Checkpoints in Cancer and Tissue Damage"
TPMA2, 2. OG
Franz-Volhard-Str. 6
68167 Mannheim
Phone +49 621 383-71509
Fax +49 621 383-71506
grk2727@medma.uni-heidelberg.de
Twitter GRK 2727: @grk2727