Sie befinden sich hier
Inhalt
Date: 02.02.2025
New Breakthrough in Glioblastoma Treatment: Engineered T Cells Offer New Hope
Glioblastoma, one of the deadliest forms of brain cancer, has long been resistant to existing treatments. However, a new study has identified a promising approach using genetically engineered T cells to specifically target and destroy glioblastoma cells. Researchers have discovered that a protein known as PTPRZ1, which is present in glioblastoma stem cells, can be effectively targeted by specialized immune cells called T cell receptor-engineered T cells (TCR-T). This discovery could lead to a major advance in the treatment of glioblastoma.
How the New Therapy Works
TCR-T cell therapy involves directing a patient's own immune cells to recognize and attack cancer cells. In this study, scientists retrieved a powerful tumor-targeting T cell receptor (TCR) from a glioblastoma patient who had previously received a cancer vaccine. This TCR was then engineered into T cells, creating PTPRZ1-specific TCR-T (PTPRZ1-TCR-T) cells capable of recognizing and killing glioblastoma cells with high precision.
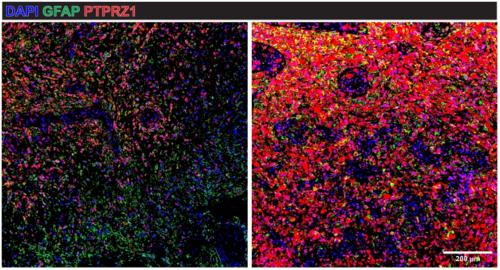
By analyzing primary brain tumor samples, the researchers confirmed that PTPRZ1 is widely overexpressed in glioblastoma cells, particularly in glioblastoma stem cells. These cells are known to drive tumor growth and resistance to treatment, making them a critical target in the fight against the disease. Importantly, the engineered TCR-T cells were shown to kill glioblastoma cells without affecting healthy brain tissue, demonstrating strong potential for safe use in patients.
Preclinical Success and Future Clinical Trials
Laboratory tests and experimental brain tumor models showed that PTPRZ1-TCR-T cells effectively eliminated glioblastoma cells. When administered both intravenously and directly into the brain, these engineered cells significantly reduced tumor growth, offering a powerful new strategy for tackling this aggressive cancer. Furthermore, the TCR-T cells maintained their efficacy over time and exhibited a "stem cell memory phenotype"—a key feature that may enable long-lasting immunity against tumor recurrence.
One of the key advantages of TCR-T cell therapy over other immune-based treatments, such as CAR-T cell therapy, is its ability to recognize and attack intracellular tumor proteins. While CAR-T therapy has shown success in blood cancers, it has been less effective in solid tumors such as glioblastoma due to challenges in targeting specific cancer markers. This study demonstrates that TCR-T therapy can overcome these limitations by precisely identifying and eliminating glioblastoma cells while sparing normal brain cells.
Encouraged by these promising results, researchers are now preparing for a first-in-human clinical trial called INVENT4GB. This phase I trial will evaluate the safety and feasibility of PTPRZ1-TCR-T therapy in patients with recurrent glioblastoma. If successful, it could pave the way for a new class of personalized immunotherapies for brain tumors.
A Step Toward Personalized Medicine
Glioblastoma remains a challenging disease with limited treatment options, but this study offers new hope for patients. By harnessing the power of the immune system to specifically target tumor cells, TCR-T therapy has the potential to revolutionize glioblastoma treatment and extend patients' lives.
While more research is needed to refine and expand this approach, this breakthrough represents an exciting step toward personalized medicine, where treatments are tailored to the unique molecular profile of each patient's tumor. Scientists are also investigating whether this therapy could be adapted to other brain tumors and cancers that express PTPRZ1, further expanding its impact.
Original scientific publications:
Vaccine-induced T cell receptor T cell therapy targeting a glioblastoma stemness antigen.
Chih YC, Dietsch AC, Koopmann P, Ma X, Agardy DA, Zhao B, De Roia A, Kourtesakis A, Kilian M, Krämer C, Suwala AK, Stenzinger M, Boenig H, Blum A, Pienkowski VM, Aman K, Becker JP, Feldmann H, Bunse T, Harbottle R, Riemer AB, Liu HK, Etminan N, Sahm F, Ratliff M, Wick W, Platten M, Green EW, Bunse L.
Nat Commun. 2025 Feb 1;16(1):1262.
DOI: 10.1038/s41467-025-56547-w