Sie befinden sich hier
Inhalt

Mannheim University of Applied Sciences
Professor of Bioanalytics and Drug Discovery,
Head, Institute of Instrumental Analytics and Bioanalytics
Head of CeMOS (Center for Mass Spectrometry and Optical Spectroscopy)
Enabling Technology Platforms for Neurodegeneration Research and Translational Neuroscience
We develop spatially resolved metabolomics, lipidomics, glycomics, and proteomics technologies and apply them with partners to multiple research topics in neurooncology and neurodegeneration research.
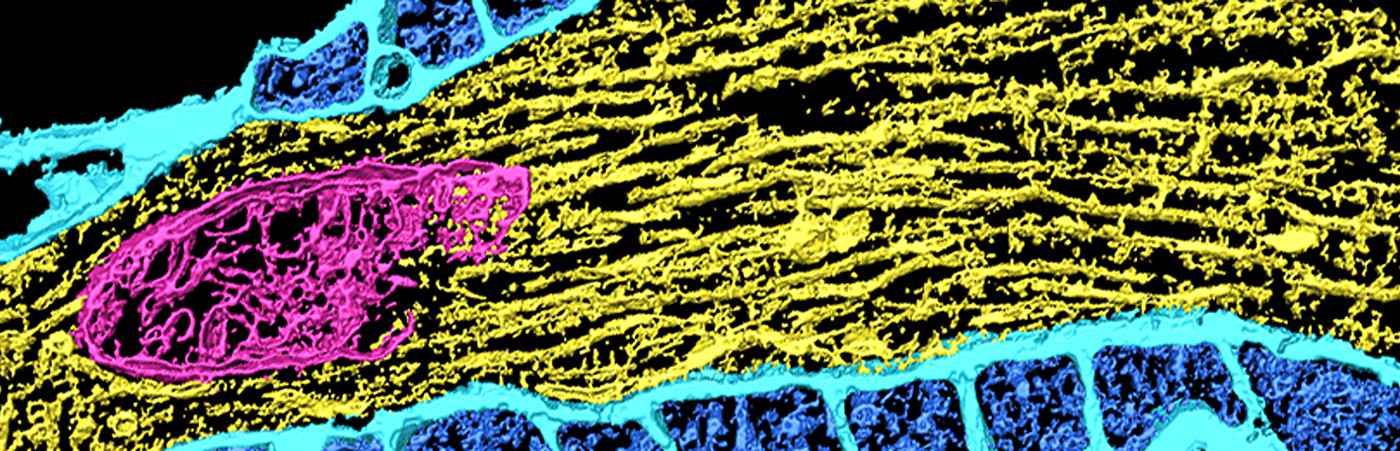
For example, protein aggregates such as deposits of β-amyloid peptides and associated lipids contribute to the pathogenesis of Alzheimer's disease (AD) and other neurodegenerative diseases. Our mass spectrometry imaging (MSI) platform can visualize their exact molecular composition in a spatial 3D-context. Increasingly, lipids, e.g. from microglial exosomes, or disturbances in lipid homeostasis are also being linked to the pathogenesis of neurodegenerative diseases. We therefore develop multimodal lipid, drug and protein imaging methods and IT-packages.
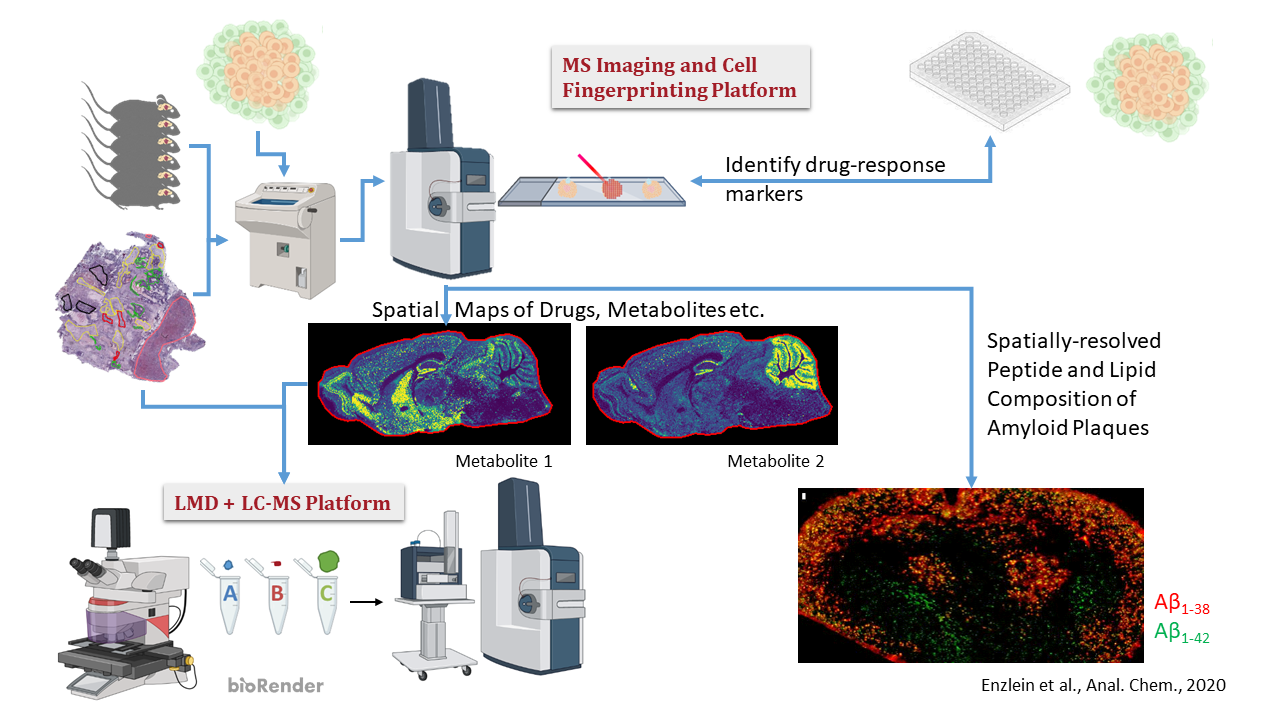
MS imaging in general enables label-free simultaneous spatially resolved (5-50 µm) imaging of thousands of molecules (drugs, metabolites, peptides, lipids) in tissue sections that can be 3D-reconstructed. It can be used for imaging known molecules, but also for unbiased computer-aided search for small molecule biomarkers.
Our second major technology platform, MS-based cell assays and single-cell lipid/metabolite fingerprinting, also does not require reagents (such as antibodies, fluorophores) and thus avoid many artifacts of conventional assays in drug discovery. Approaching single-cell resolution, we analyse the molecular content of cells and their change in response to stimuli and drug-like compounds using AI/machine learning approaches.
The current focus of this work is:
- Mass spectrometric imaging: we are exploring and developing with partners i) new auxiliary chemicals for selective analysis of specific classes of molecules, e.g. lipids, ii) new mass spectrometric techniques, especially by combining them with other imaging modalities (e.g. infrared microscopy), iii) new ML-enabled IT solutions for image processing and targeted biomarker discovery, and iv) new devices to support this technology. Current Neuroscience Examples: Visualization of the distribution of neuro-oncometabolites like 2-HG or Trp catabolites in patient tissues or the automated computational analysis of AD amyloid plaque composition.
- Mass spectrometric assays: The focus here is on complex biochemical assays in work with partners, e.g. based on cell-free preparations of the gamma-secretase of Alzheimer's disease, and cell-based assays, e.g. for substances that modulate lipid metabolism or drug uptake.
- Developing new Mass Spectrometry technology, currently on Bruker Autoflex Speed, UltrafleXtreme, RapifleX, timsTOFflex (ion mobility MS) and solarix (magnetic resonance MS) mass spectrometers in conjunction with infrared imaging, laser capture microdissection.
National and international joint research projects
- BMBF: SMART-CARE-2 – A systems medicine approach to stratify cancer recurrence; Duration: 2023 – 2026
- MWK: Collaborative Research Training Group Perpharmance - Personalized Medicine and Organoid Pharmaceutical Test Models: Advanced Materials, Analytics, and Computing; since 2023
- Subproject: Deep profiling of complex tissues via MS- and Infrared-Imaging
- DFG: Integrative Analysis of Organoids Enables Mechanistic Studies in Pharmaceutical Research: Development, Volatilomics, Single Cell Metabolite/Lipid Fingerprinting, and Function; Duration: 2022-2027
- BMBF: SMART-CARE – A systems medicine approach to stratify cancer recurrence; Duration: 2020 – 2023
- BMBF: MSCORESYS – Research Cores for Mass Spectrometry in Systems Medicine: SMART-CARE; Duration: 2020-2026
- BMWi ZIM: Project lead of „Hochauflösende MALDI-Matrizes“. Project duration: 2020 – 2022
- DFG: SFB 1389 – UNITE Glioblastoma; Duration: 2019 – 2027
- Subproject C04: Metabolic signaling in glioblastoma: a spatial multi-omics approach; 2nd funding period (in cooperation with Christiane Opitz)
- Subproject C04: Identification and spatial mapping of predictive biomarker signatures by maldi mass spectrometry imaging (MSI); 1st funding period (in cooperation with Christiane Opitz)
- EU: EU-OPENSCREEN-DRIVE: Driving forward long-term sustainability of excellence in chemical biology within Europe and beyond (H2020 call H2020-INFRADEV-2018-1); Duration: 2019 – 2023
- BMBF: Project lead of M2Aind-ADCtox-3D. Toxicological assessment of ADCs 8 ADCs (antibody-drug conjugates) in 3D cell cultures. Project duration: 2019 – 2020.
- BMBF: FH-Impuls – Spokesperson and overall scientific lead of the FH-Impuls industry partnership M2Aind (“Multimodal Analytics for the Health Industry”), Project duration: 2017 – 2020
- Subproject: SM2all - Low Molecular Active Ingredients: Production, Analysis, Safety, Efficacy
- Subproject: M2OGA - Molecular Human Organoid and Tissue Analysis: Multimodality, Digitization, 3D Imaging, Highly Integrated Applications
- BMBF: Forschungscampus Mannheim Molecular Intervention Environment: Molekulare Bioanalytik und Theranostika-Entwicklung; Duration: 2015 – 2025
Legend: DFG = German Research Foundation, BMBF = Federal Ministry of Education and Research, EU = European Union, BMWi = Federal Ministry for Economic Affairs and Energy, ZIM = Zentrales Innovationsprogramm Mittelstand (Central Innovation Program for SMEs); MWK = Ministry of Science, Research and the Arts
Selected publications
- Spatial probabilistic mapping of metabolite ensembles in mass spectrometry imaging.
Abu Sammour D, Cairns JL, Boskamp T, Marsching C, Kessler T, Ramallo Guevara C, Panitz V, Sadik A, Cordes J, Schmidt S, Mohammed SA, Rittel MF, Friedrich M, Platten M, Wolf I, von Deimling A, Opitz CA, Wick W, Hopf C. Nat Commun. 2023. 14(1):1823. doi: 10.1038/s41467-023-37394-z. - PS-induced lipid alterations in microglia revealed by MALDI mass spectrometry-based cell fingerprinting in neuroinflammation studies.
Blank M, Enzlein T, Hopf C. Sci Rep. 2022. 12(1):2908. doi: 10.1038/s41598-022-06894-1. - Structural amyloid plaque polymorphism is associated with distinct lipid accumulations revealed by trapped ion mobility mass spectrometry imaging.
Michno W, Wehrli PM, Koutarapu S, Marsching C, Minta K, Ge J, Meyer SW, Zetterberg H, Blennow K, Henkel C, Oetjen J, Hopf C, Hanrieder J. J Neurochem. 2022. 160(4):482-498. doi: 10.1111/jnc.15557. - Label-free cell assays to determine compound uptake or drug action using MALDI-TOF mass spectrometry.
Unger MS, Blank M, Enzlein T, Hopf C. Nat Protoc. 2021. 16(12):5533-5558. doi: 10.1038/s41596-021-00624-z. - Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas.
Friedrich M, Sankowski R, Bunse L, Kilian M, Green E, Ramallo Guevara C, Pusch S, Poschet G, Sanghvi K, Hahn M, Bunse T, Münch P, Gegner HM, Sonner JK, von Landenberg A, Cichon F, Aslan K, Trobisch T, Schirmer L, Abu-Sammour D, Kessler T, Ratliff M, Schrimpf D, Sahm F, Hopf C, Heiland DH, Schnell O, Beck J, Böttcher C, Fernandez-Zapata C, Priller J, Heiland S, Gutcher I, Quintana FJ, von Deimling A, Wick W, Prinz M, Platten M. Nat Cancer. 2021. 2(7):723-740. doi: 10.1038/s43018-021-00201-z. - Following spatial Aβ aggregation dynamics in evolving Alzheimer's disease pathology by imaging stable isotope labeling kinetics.
Michno W, Stringer KM, Enzlein T, Passarelli MK, Escrig S, Vitanova K, Wood J, Blennow K, Zetterberg H, Meibom A, Hopf C, Edwards FA, Hanrieder J. Science Adv. 2021. 7(25):eabg4855. doi: 10.1126/sciadv.abg4855. - Computational Analysis of Alzheimer Amyloid Plaque Composition in 2D- and Elastically Reconstructed 3D-MALDI MS Images.
Enzlein T, Cordes J, Munteanu B, Michno W, Serneels L, De Strooper B, Hanrieder J, Wolf I, Chávez-Gutiérrez L, Hopf C. Anal Chem. 2020. 92(21):14484-14493. doi: 10.1021/acs.analchem.0c02585. - Direct Automated MALDI Mass Spectrometry Analysis of Cellular Transporter Function: Inhibition of OATP2B1 Uptake by 294 Drugs.
Unger MS, Schumacher L, Enzlein T, Weigt D, Zamek-Gliszczynski MJ, Schwab M, Nies AT, Drewes G, Schulz S, Reinhard FBM, Hopf C. Anal Chem. 2020. 92(17):11851-11859. - Mechanistic MALDI-TOF Cell-Based Assay for the Discovery of Potent and Specific Fatty Acid Synthase Inhibitors.
Weigt D, Parrish CA, Krueger JA, Oleykowski CA, Rendina AR, Hopf C. Cell Chem Biol. 2019. 26(9):1322-1331.e4. doi: 10.1016/j.chembiol.2019.06.004. - Alzheimer's-Causing Mutations Shift Aβ Length by Destabilizing γ-Secretase-Aβn Interactions.
Szaruga M, Munteanu B, Lismont S, Veugelen S, Horré K, Mercken M, Saido TC, Ryan NS, De Vos T, Savvides SN, Gallardo R, Schymkowitz J, Rousseau F, Fox NC, Hopf C, De Strooper B, Chávez-Gutiérrez L. Cell. 2017. 170(3):443-456.
Kontextspalte
Contact
Hochschule Mannheim
CeMOS
Paul-Wittsack-Str. 10
68163 Mannheim
Phone +49 621 292-6802
c.hopf@hs-mannheim.de