Sie befinden sich hier
Inhalt

Central Institute of Mental Health
Heisenberg Professor of Molecular and Cellular Cognition Research
Department Molecular and Cellular Cognition Research
Uncover the molecular and cellular processes that regulate memory formation and persistence
Our overall goal is to investigate the molecular and cellular mechanisms underlying cognitive processes and neuroplastic changes associated with psychiatric disorders.
Neuroplasticity allows the brain to continuously adapt its functional and structural organization to changing environments. This ability is essential for the individual’s survival, as it allows the adaptation to the surroundings and underlies key functions such as learning and memory. However, the same mechanism can turn into maladaptive changes that give rise to pathological conditions. Despite advances in the field, the molecular mechanisms of neuroplasticity in health and disease remain poorly understood. The overall goal of the department is to fill this knowledge gap.
We focus on investigating the mechanisms that govern neuroplasticity in the context of memory formation and persistence and their dysfunction in psychiatric conditions. In particular, we are interested in investigating the molecular and cellular basis of the protracted functional and morphological changes triggered by aversive environmental factors (e.g. trauma, substance use, noxious stimuli) that contribute to mental health disorders. The targeting of neuroplasticity processes promises to achieve therapeutic strategies. Targeting neuroplastic processes holds great promise for therapeutic strategies. Particular attention is paid to transcriptional and epigenetic regulation, given their key role in regulating long-term adaptations.
We take an interdisciplinary approach that utilizes behavioral testing, engram tools, chemogenetics, epigenetic and transcriptional analysis, as well as morphological and electrophysiological approaches.
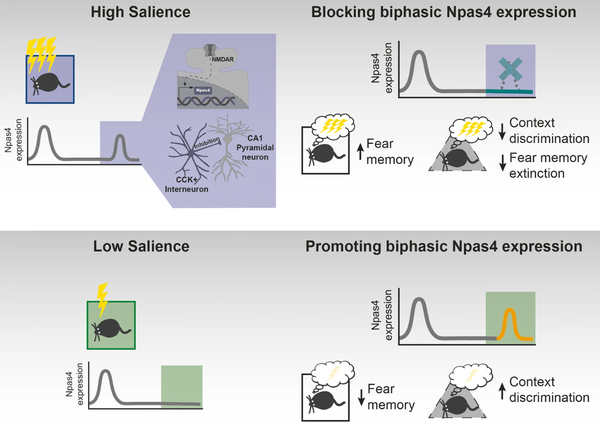
Figure: Role of Npas4 biphasic expression for memory consolidation. Upper part: High salience learning (purple) induces Npas4 biphasic expression in the CA1 region of the hippocampus in a NMDAR-dependent manner and increases CCK+ IN inputs. Pharmacologically blocking NMDAR activity, and consequently late Npas4 expression, results in enhanced fear memory, reduced context discrimination and impairs fear memory extinction. Nonetheless, it should be noted that other mechanisms besides Npas4 expression occur due to NMDAR activation and may contribute to memory suppression. Lower part: Low salience learning (green) leads to one peak of Npas4 expression. Genetically promoting a biphasic expression of Npas4 results in reduced fear memory and increased context discrimination.
From: Biphasic Npas4 expression promotes inhibitory plasticity and suppression of fear memory consolidation in mice. Brito et al., Mol Psychiatry, 2024
Research projects
DFG: ”Characterization and reversibility of cognitive deficits in a mouse model of Tatton-Brown-Rahman syndrome.”, Duration: since 2023
DFG: ”Molecular mechanisms of memory persistency”, Duration: since 2021
DFG:“Epigenetic control of long-lasting cortical memory representations”, Duration: since 2021
Legend: DFG = German Research Foundation
Selected Publications
- Biphasic Npas4 expression promotes inhibitory plasticity and suppression of fear memory consolidation in mice.
Brito DVC, Kupke J, Sokolov R, Cambridge S, Both M, Bengtson CP, Rozov A, Oliveira AMM. Mol Psychiatry. 2024 Feb 13. doi: 10.1038/s41380-024-02454-3 - Regulation of neuronal plasticity by the DNA repair associated Gadd45 proteins.
Brito DVC, Kupke J, Gulmez Karaca K, Oliveira AMM. Current Research in Neurobiology. 2022. 3:100031. doi: 10.1016/j.crneur.2022.100031 - Engram reactivation during memory retrieval predicts long-term memory performance in aged mice.
Gulmez Karaca K, Brito DCV, Kupke J, Zeuch B, Oliveira AMM. Neurobiology of Aging. 2021. 101:256-261. doi: 10.1016/j.neurobiolaging.2021.01.019 - Neuronal ensemble-specific DNA methylation strengthens engram stability.
Gulmez Karaca K, Kupke J, Brito DVC, Zeuch B, Thome C, Weichenhan D, Lutsik P, Plass C, Oliveira A. Nat Commun. 2020. 11(1):639. doi: 10.1038/s41467-020-14498-4 - Mimicking Age-Associated Gadd45γ Dysregulation Results in Memory Impairments in Young Adult Mice.
Brito DVC, Kupke J, Gulmez Karaca K, Zeuch B, Oliveira A. J Neurosci. 2020. 40(6):1197-1210. doi: 10.1523/JNEUROSCI.1621-19.2019 - Dnmt3a2: a hub for enhancing cognitive functions.
Oliveira A, Hemstedt TJ, Freitag HE, Bading H. Mol Psychiatry. 2016. 21(8):1130-6. doi: 10.1038/mp.2015.175 - Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities.
Oliveira A, Hemstedt TJ, Bading H. Nat Neurosci. 2012. 15(8):1111-3. doi: 10.1038/nn.3151 - Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation.
Mauceri D, Freitag HE, Oliveira A, Bengtson CP, Bading H. Neuron. 2011. 71(1):117-30. doi: 10.1016/j.neuron.2011.04.022
Kontextspalte
Contact
Medical Faculty Mannheim
Heidelberg University
Theodor-Kutzer-Ufer 1-3
68167 Mannheim