Sie befinden sich hier
Inhalt
L. R. Schad, F. Wenz, M. V. Knopp, K. Baudendistel, E.Müller, W. J. Lorenz
Introduction
At the 11. SMRM meeting 1992 in Berlin recently reported studies were presented on completely non invasive MR imaging of human brain activity based on either blood flow or blood oxygenation changes (1-13). Most of the investigators were using EPI methods requiring special hardware equipment. Stimulation measurements on standard scanners using conventional imaging techniques were reported rarely and are essentially preliminary (14, 15). The present study was undertaken to evaluate the feasibility of functional imaging of the human brain on the basis of conventional FLASH sequences using a standard 1.5 T whole body equipment.
Methods
2D and 3D functional imaging of motor cortex stimulation of 20 normal volunteers was performed on a standard 1.5T clinical scanner (Siemens, Magnetom). A circular polarized head coil has been used for the stimulation measurements, although improvement in S/N ratio has been achieved by using a commercially available eye/ear surface coil with a loop of 8.5cm in diameter. The imaging technique used was a conventional 2D and 3D, first-order flow rephased, FLASH sequence with fat-suppression and reduced bandwidth (16-28 Hz/pixel) and TR=80-120ms, TE=60ms, flip angle=40°, matrix=128x128, FOV=150-250mm, slice-thickness=2-5mm, NEX=1, and a total single scan time of about 12-20sec. The motor cortex stimulation was achieved by touching each finger to thumb in a sequential, self-paced, and repetitive manner.
Results
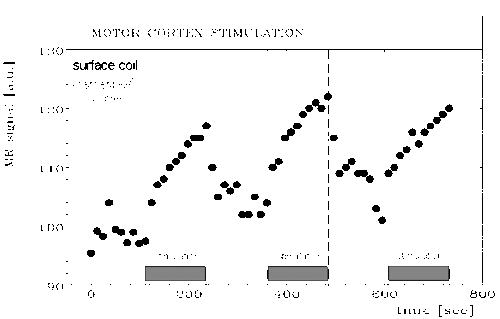
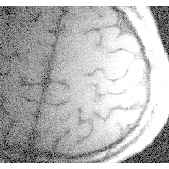
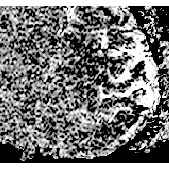
During stimulation an increase in signal occurred in areas predominantly occupied by the grey matter of primary motor and sensory cortex (head coil: 5-10% effect, surface coil: 10-20% effect). The effect in signal change during stimulation can be clearly seen in one pixel only with an increase in signal of order 20%. Different signal behaviour during stimulation was observed, where most of the volunteers showed a more or less constant enhanced signal during stimulation but in some cases a clear linear signal increase was observed (Fig. 1). High resolution 3D FLASH imaging (slap=32mm, partitions=16) showed a good anatomic correlation of signal increase and grey matter in areas of primary motor and sensory cortex (Fig. 2)
Conclusions
The observed increase in signal intensity in the activated brain areas is potentially explained qualitatively by findings previously confirmed by PET observations. Thereby deoxyhemoglobin acts as an effective endogenous contrast agent, where signal variation during stimulation is caused by differences in the magnetic susceptibility of blood induced by the presence of paramagnetic deoxyhemoglobin in red cells (1-13). High spatial resolution, improved S/N ratio, reduced bandwidth, and fat-suppression resulted in a clear enhancement of the observed stimulation effect (about 20%) and an improvement in detectability due to reduced partial volume effect. Our data (20 volunteers) show different signal behaviour during stimulation. At the moment the neurophysiological explanation of this observation is unclear and needs some further clinical investigations completed by PET experiments.
In conclusion, our data demonstrate the technical feasibility of 2D and 3D functional MR imaging at high spatial resolution on a standard clinical 1.5T scanner using optimized gradient echo sequences and coil equipment. 2D time course gradient echo imaging is a powerful non invasive tool for assessment of regional cerebral activation with high temporal and spatial resolution. 3D functional imaging is another powerful tool to image the stimulation effects of the whole brain cortex and a maximum intensity projection (MIP), the well known projection method in MR angiography, seems to be possible.
References
[1 - 13] Society of Magnetic Resonance in Medicine (SMRM), Eleventh Annual Meeting, Berlin, Book of Abstracts, ISSN 0891-7612, 203, 301, 302, 304, 719, 1820, 1822, 1823, 1824, 1825, 1826, 1827, 1834, (1992).
[14] Frahm, J.; Merbold, K.D.; Hänicke, W. Functional MRI of human brain activation at high spatial resolution. Mag Reson Med, 29(1), 139-144, (1993).
[15] Schad, L.R.; Trost, U.; Knopp, M.V.; Müller, E.; Lorenz, W.J. Motor cortex stimulation measured by magnetic resonance imaging on a standard 1.5 Tesla clinical scanner. Mag Res Imag, 11(4), (1993) in press.