Sie befinden sich hier
Inhalt

Neurophysiology, Pain, Itch
Professor of Anesthesiology
Translational pain research
In this research group sensitization of primary afferent nociceptors is investigated as one mechanism for inflammatory and neuropathic chronic pain. We focus on translational studies using single fiber recording techniques in chronic pain patients, human volunteers and large animals (pig) combined with functional and structural investigations of skin innervation in-vivo and in cell-culture.
- Cellular porcine model
Patch-clamp and histochemistry of porcine dorsol root ganglion cells to characterize different nociceptor classes. Development of in-vitro model of nerve endings separated from the cell body and coculture with keratinocytes. - Porcine single fiber recordings
Characterization of different afferent nerve fiber classes and modulation of axonal excitability using standard teased fiber techniques in the pig saphenous nerve. Effects of locally applied growth factors on excitability of nerve endings and axonal excitability. - Porcine neurogenic inflammation
Development of experimental models of peripheral sensitization by UVB irradiation with the objective readout of axon reflex erythema measured by laser Doppler imaging. Microdialysis in the sunburn to assess levels of inflammatory mediators involved in peripheral sensitization. - Human pain models
Characterization of human pain models for peripheral (UVB irradiation) and central (electrical pain and hyperalgesia model) sensitization by pharmacological intervention. Objective assessment of cutaneous unmyelinated innervation by electrically induced axon reflex erythema and electrically induced axon reflex sweating. - Chronic pain patients
Microneurographic characterization of neuropathic changes in primary afferent fibers linked to chronic pain. Correlation of functional and structural changes in neuropathic pain by using skin biopsies, quantitative sensory testing, and electrically induced axon reflex measurements.
National and international joint research projects
DFG: Heidelberg Pain Consortium SFB 1158: “From nociception to chronic pain: Structure- function properties of neural pathways and their reorganization” / 3rd funding period, Duration: 2023 – 2027
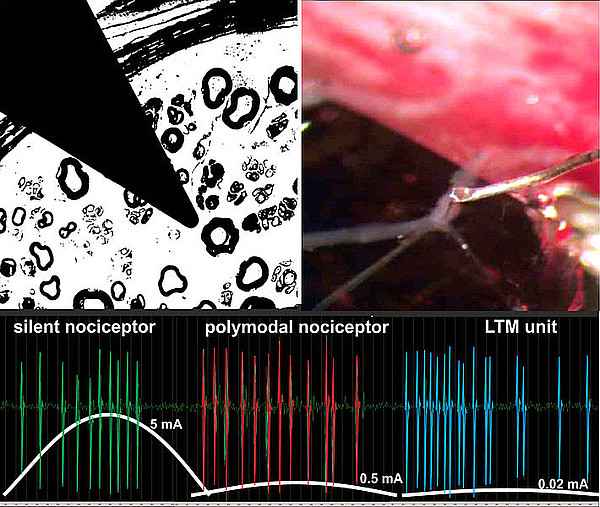
- Project A01: “Silent nociceptors in mice and humans”
DFG: Heidelberg Pain Consortium SFB 1158: “From nociception to chronic pain: Structure- function properties of neural pathways and their reorganization” / 2nd funding period, Duration: 2019 – 2023
- Project A01: “silent nociceptors in mice and humans”
DFG: Speaker of the Research Group FOR 2690: "Translational Pruritus Research" / 1st and 2nd funding period, Duration: 2018 – 2024
DFG: International Research Training Group RTG 1874 "DIAMICOM (Diabetic Microvascular Complications)”, Duration: 2017 – 2021
- Project MD SP14: “Diabetic thin fiber neuropathy – objective assessment and mechanisms”
Legend: DFG = German Research Foundation
Selected publications
- Optimized Electrical Stimulation of C-Nociceptors in Humans Based on the Chronaxie of Porcine C-Fibers.
Schneider T, Filip J, Soares S, Sohns K, Carr R, Rukwied R, Schmelz M. (2023) J Pain 24(6):957-969. - How Do Neurons Signal Itch?
Schmelz M. (2021) Front Med (Lausanne) 8:643006. - Slow depolarizing stimuli differentially activate mechanosensitive and silent C nociceptors in human and pig skin.
Rukwied R, Thomas C, Obreja O, Werland F, Kleggetveit IP, Jorum E, Carr RW, Namer B, Schmelz M. (2020) Pain 161(9):2119-2128. - TTX-resistant sodium channels functionally separate silent from polymodal C-nociceptors.
Jonas R, Prato V, Lechner SG, Groen G, Obreja O, Werland F, Rukwied R, Klusch A, Petersen M, Carr RW, Schmelz M. (2020) Front Cell Neurosci 14:13. - Nerve growth factor locally sensitizes nociceptors in human skin.
Obreja O, Rukwied R, Nagler L, Schmidt M, Schmelz M, Namer B. (2018) Pain 159(3):416-426. - Low-Frequency Stimulation of Silent Nociceptors Induces Secondary Mechanical Hyperalgesia in Human Skin.
Sauerstein K, Liebelt J, Namer B, Schmidt R, Rukwied R, Schmelz M. (2018) Neuroscience 387:4-12. - Clinical presentation, management, and pathophysiology of neuropathic itch.
Steinhoff M, Schmelz M, Szabo IL, Oaklander AL. (2018) Lancet Neurol 17(8):709-720. - Tuning in C-nociceptors to reveal mechanisms in chronic neuropathic pain.
Jonas R, Namer B, Stockinger L, Chisholm K, Schnakenberg M, Landmann G, Kucharczyk M, Konrad C, Schmidt R, Carr R, McMahon S, Schmelz M, Rukwied R. (2018) Ann Neurol 83(5):945-957. - Specific changes in conduction velocity recovery cycles of single nociceptors in a patient with erythromelalgia with the I848T gain-of-function mutation of Nav1.7.
Namer B, Orstavik K, Schmidt R, Kleggetveit IP, Weidner C, Mork C, Kvernebo MS, Kvernebo K, Salter H, Carr TH, Segerdahl M, Quiding H, Waxman SG, Handwerker HO, Torebjork HE, Jorum E, Schmelz M. (2015) Pain 156(9):1637-1646. - The neurobiology of itch.
Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. (2006) Nat Rev Neurosci 7(7):535-47. - Itch.
Yosipovitch G, Greaves MW, Schmelz M. (2003) The Lancet 361(9358):690-694.
Kontextspalte
Contact
Medical Faculty Mannheim
Heidelberg University
Ludolf-Krehl-Str. 13-17
68167 Mannheim
Phone +49 621 383-71650
martin.schmelz@medma.uni-heidelberg.de