Sie befinden sich hier
Inhalt
Date: 04.06.2024
Inclusion body myositis (IBM) is a chronic, insidiously progressive inflammatory muscle disease that is also associated with degenerative processes - with muscle atrophy, particularly in the arms and legs. The causes and mechanisms of disease progression are still largely unknown. As a result, there is no effective therapy. Inclusion body myositis is considered the most common inflammatory muscle disease in the elderly. Therefore, it is of great interest to gain new insights into its pathomechanisms and to identify possible therapeutic targets.
An international study led by scientists from the Division of Neuroimmunology in the Department of Neurology at the University Medical Center Mannheim (UMM) and the Myositis Center at Johns Hopkins Medical School, has provided valuable new insights into the molecular changes associated with the pathogenesis of IBM, based on state-of-the-art single-cell RNA sequencing of muscle biopsies from patients. For example, the scientists found that certain muscle fiber types (type 2A) are particularly susceptible to the pathological processes associated with inclusion body myositis - associated with genetic stress of the cells and detectable by an increase in corresponding markers such as GADD45A, which indicates pronounced DNA damage in the cell nucleus.
In addition, the researchers found evidence of possible acetylcholinesterase (ACHE) dysfunction in the inflamed muscles of IBM patients. This enzyme plays a critical role in signaling between the nervous system and muscles. The researchers found elevated levels of a specific long non-coding RNA (lncRNA) called NORAD, which does not code for a protein. “We know from previous studies that NORAD regulates so-called pumilio proteins, which in turn can lead to increased activity of the enzyme acetylcholinesterase,” says Sven Wischnewski from the University Medical Center Mannheim, Germany, one of the lead authors of the present study.
In myasthenia gravis, an autoimmune-mediated neurological disease in which signal transmission between nerve cells and muscles is disrupted, drugs that inhibit the activity of acetylcholinesterase have been used for years to improve patients' symptoms. "The molecular changes we observed therefore fit well with the findings in the muscles of patients with disorders of signal transmission between nerve and muscle," adds Sven Wischnewski.
"With this study, we have identified new and potentially treatable mechanisms that could help to protect damaged muscle fibers in the long term and promote their resistance," says Lucas Schirmer, Head of the Division of Neuroimmunology in the Department of Neurology at the UMM, who is one of the corresponding authors of the current study together with Thomas E. Lloyd (Baylor College of Medicine / Johns Hopkins Medical School). The findings are important for a better understanding of the molecular disease mechanisms of inclusion body myositis in particular. They also offer new therapeutic approaches for inflammatory muscle diseases in general.
In addition to the researchers at the UMM and the Johns Hopkins Medical School, scientists from the University Medical Centers in Mainz, Ulm and Berlin, Germany, as well as the University of California in Santa Cruz, USA, were involved in the study.
Source: press release UMM

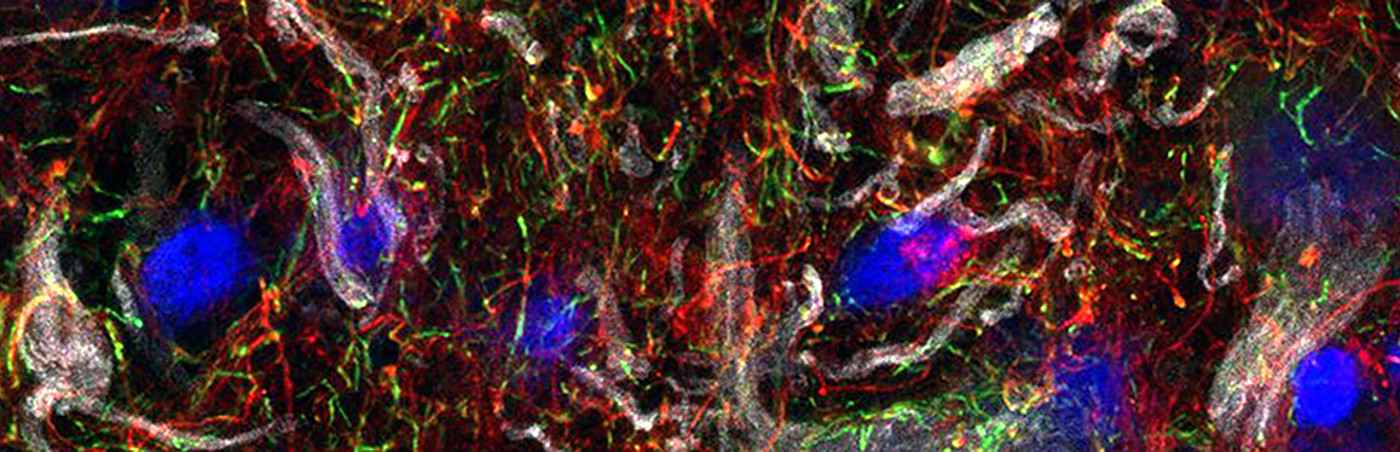
Figure: The fluorescence image (RNA in situ hybridization) shows the increased presence of the lncRNA NORAD (white), ACHE (green) and the DNA damage marker GADD45A (magenta) in inflamed muscles in IBM. Nuclei are shown in blue. The frequent appearance of NORAD and ACHE may represent a new approach for the treatment of IBM.
Press release of the University Medical Centre Mannheim (German language):
04.06.2024 Neue Erkenntnisse und Therapieansätze bei entzündlichen Muskelerkrankungen
Original scientific publications:
Cell type mapping of inflammatory muscle diseases highlights selective myofiber vulnerability in inclusion body myositis.
Wischnewski S, Thäwel T, Ikenaga C, Kocharyan A, Lerma-Martin C, Zulji A, Rausch HW, Brenner D, Thomas L, Kutza M, Wick B, Trobisch T, Preusse C, Haeussler M, Leipe J, Ludolph A, Rosenbohm A, Hoke Ahmet, Platten M, Weishaupt JH, Sommer CJ, Stenzel W, Lloyd TE, Schirmer L.
Nature Aging. 2024 June 4
DOI: 10.1038/s43587-024-00645-9