Sie befinden sich hier
Inhalt
Improved Target Volume Definition in Radiosurgery of Brain Metastases by Stereotactic Correlation of Morphological and Functional MRI.
L. R. Schad, M. Bock, M. Günther, J. Hirsch, J. Reichenbach, H. Schlemmer, K. Eichhorn, J. Debus
Introduction
In the past decade dynamic MRI techniques have been developed to study the physiological properties of human tissue (perfusion, diffusion, BOLD). Morphology and function can be evaluated and assessed qualitatively as well as quantitatively using MRI in stereotactic treatment planning and monitoring of patients with brain lesions. This can be achieved by accurate image correlation using a stereotactic head frame. The aim of the present study is a further improvement in the definition of the target volume by direct correlation of physiological MRI-data with morphology [1]. In addition, the effects of radiosurgery on tissue perfusion and oxygenation level can be monitored by precise stereotactic correlation with the dose distribution.
Material and methods
In a clinical pilot study 20 patients with brain metastases were treated and monitored after high dose radiosurgery. An individual mask in combination with a stereotactic localization system fitting properly into the head coil was used in MRI for accurate and reproducible positioning of the patient’s head. MRI was performed to assess morphology (T1-, T2-weighted, and diffusion imaging), tissue perfusion (spin-labeling technique), angiography (subtraction of prae and post Gd-DTPA imaging), and venography (BOLD-technique). A flow compensated 3D FLASH technique was used to assess both tissue and vessel morphology: TR = 20 ms, TE = 6.5 ms, FL = 25°, FOV = 270 mm2, MA = 384x512, TH = 3 mm, 56 slices, acquisition time 7 min. CSF-suppressed diffusion imaging was obtained with a spin-echo EPI sequence in a single acquisition scheme. The EPI-FLAIR technique [2] with four b-values ranging from 0.16 to 1000 s/mm2 allowing isotrope ADC parameter maps with MA = 96x128, TH = 6 mm, 9 slices, acquisition time 1.5 min. Perfusion weighted imaging was achieved by the EPI-FAIR technique [3] at an inversion time of TI = 1500 ms and 64 acquisitions with MA = 128x128, TH = 9 mm, 5 slices, acquisition time 5 min. Finally high-resolution MR venography [4] was measured using a T2*-weighted 3D FLASH technique: TR = 42 ms, TE = 25 ms, MA = 384x512, TH = 3 mm, 32 slices, acquisition time 8 min. All measurements were implemented on a clinical imager (1.5 T Magnetom VISION, SIEMENS, Erlangen, Germany) equipped with resonant EPI hardware, i.e. maximum gradient strength of 25 mT/m and rise time of 300 µs. Total examination time was about 45 min.
Results and conclusions
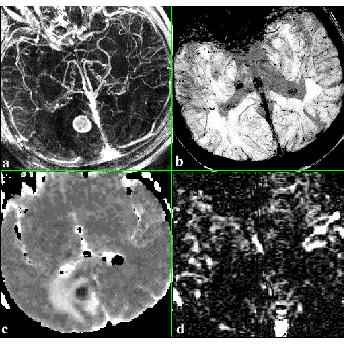
Fig. 1 shows a 49 year old patient with a brain metastases before treatment. Angiographic information of both the arteries and veins can be evaluated by a maximum intensity projection (MIP) of the subtracted prae/post Gd-DTPA images as well as rCBV values of normal tissue (Fig. 1a). To create the high-resolution venograms a minimum intensity projection (mIP) was performed over targeted volumes. Separation of veins from arteries was achieved by multiplying the magnitude images with a phase mask filter constructed from the phase images prior to the projection. Exploiting the small but finite difference in magnetic susceptibility between venous and arterial blood allows to visualize venous information independently from arterial information using T2*-weighted high-resolution 3D scans (Fig. 1b). The MR venogram depicts the venous part of the lesion in much greater detail for studying changes in the venous pattern at high dose radiotherapy. The subtraction of selective and nonselective EPI-FAIR images (perfusion weighted image, Fig. 1d) shows a large area of minor tissue perfusion corresponding to the edema seen in the T2-weighted image or ADC parameter image (Fig. 1c). These different MR techniques are a useful completion in our treatment protocol for planning of radiosurgery and follow-up of brain metastases for a better understanding of the dynamic changes in physiological and morphological properties of brain lesions after radiation.
References
[1] Schad LR et al. MRI 12(5):811-819; 1994.
[2] Hajnal JV et al. JCAT 16 (6):841-844; 1992.
[3] Kim SG et al. MRM 34: 293-301; 1995.
[4] Reichenbach JR et al. Radiology 204:272-277; 1997.