Sie befinden sich hier
Inhalt
Immune interactions in diabetes and vascular complications
Diabetes became a major epidemic of this century with rapidly increasing incidence worldwide. Both type 1 diabetes (T1D) and type 2 diabetes (T2D) are clinically characterised by chronic elevation of blood sugar (hyperglycaemia). Diabetes is associated with a number of macro- and microvascular complications leading to the failure of organs such as the heart, kidneys and eyes. Hyperglycaemia activates both oxidative stress and inflammatory pathways leading to the progression of diabetes and vascular complications.
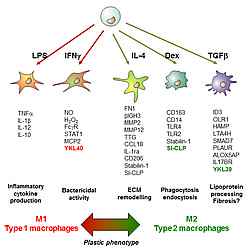
Macrophages are essential immune regulators that undergo two major vectors of polarisation: classically activated macrophages (M1) and alternatively activated macrophages (M2). Both types of macrophages can have detrimental effects on activation of various cells types including endothelial cell, platelets, and adaptive immune cells leading to the development of diabetes-associated inflammation and diabetic vascular complications. Essential features of macrophages include their functional and phenotype diversity and plasticity that makes them attractive targets for the therapeutic programming.
We have recently demonstrated that hyperglycaemia itself, without additional metabolic factors induces mixed M1/M2 cytokine profile that can support diabetes-associated inflammation and vascular complications. Our goal is to identify the mechanism of long-term macrophage programming in metabolic conditions and to develop the strategy for the therapeutic macrophage reprogramming to prevent development of diabetic vascular complications.
Current Projects
- Mechanisms of histone-code mediated hyperglycemic memory in metabolic inflammation and microvascular complications. GRK1874/2 DIAMICOM
- Effect of hyperglycemia on Toll-like receptor-mediated inflammation.
- Macrophage-platelets interaction in diabetic conditions (in cooperation with AG Prof. Bugert)
Macrophages as tools and therapeutic target in implantation and regenerative medicine
Implants, transplants, and implantable biomedical devices are mainstream solutions for a wide variety of human pathologies. Titanium and titanium alloys have been successfully used in orthopedics, dentistry, cardiology and otorhinolaryngology. Very good mechanical properties, corrosion resistance, low magnetic susceptibility and high biocompatibility have been the reason for choosing titanium for implantation. However, titanium implants can fail due to the development of various complications.
During the initial phase, proinflammatory macrophages induce acute reactions to trauma and foreign materials, whereas tolerogenic anti-inflammatory macrophages control resolution of inflammation and induce the subsequent healing stage. However, failure of macrophages to resolve the inflammation and their tendency to stay in a state of "frustrated phagocytosis leads to the chronic inflammation accompanied by microbial contamination and resulting in the implant failure.
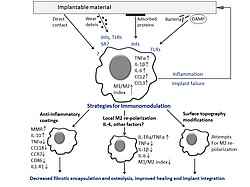
Several materials based on natural polymers for improved interaction with host tissue or surfaces that release anti-inflammatory drugs/bioactive agents have been developed for implant coating to reduce implant rejection. However, no definitive, long-term solution to avoid adverse immune responses to the implanted materials is available to date. Prevention of implant-associated infections or chronic inflammation by manipulating the macrophage phenotype is a promising strategy to improve implant acceptance.
The strategies for the control of local macrophage phenotypes include improvement of implant surface by implant coating or surface topography modifications, and development of cell therapy approaches based on the long-term fixation of macrophage phenotypes. Moreover, primary macrophages exposed to the implantable materials ex vivo can be used to predict the individual patients’ reactions and allow selection of an optimal coating composition.
Current projects
- Systems immunology analysis of macrophage responses to titanium and coating materials (EU FP7 IMMODGEL)
- Anti-inflammatory macrophages for implants and regenerative medicine (EU FP7 IMMODGEL)
- Detrimental macrophage differentiation in heart failure patient undergoing therapy with left ventricular assist device
Tumor-associated macrophages: biomarkers and therapeutic targeting
Despite significant advance in our understanding of the fundamental aspects of cancer cell biology and development of novel therapeutic tools, cancer remains to be one of most significant health problem world-wide. Growing body of evidence indicates that tumor microenvironment provides a crucial support for the primary tumor growth, invasiveness and aggressiveness of metastatic disease. Tumour-associated macrophages (TAM) is a key immune regulatory component of tumour microenvironment (Qian, Cell 2010). TAM constitute up to 50% of tumour mass and support growth of primary tumour, angiogenesis and metastatic spread (Noy, Immunity 2014).
Studies in mouse models have demonstrated that elimination of TAM by vaccination against TAM antigens results in tumour regression and abrogation of metastasis. However, this TAM-killing vaccination strategy is impossible to translate to human patients due to multiple life-threatening side effects. Advanced clinically applicable strategy for TAM targeting is their functional reprogramming (Wynn et al, Nature, 2013).
Current projects
- Biological functions and role of YKL-39 in cancer progression
- Alphavirus-based delivery of chitinase-like-protein gene for anti-tumour programing of macrophages (ERANET-Rus plus)
Selected Publications
- Riabov V, Salazar F, Htwe SS, Gudima A, Schmuttermaier C, Barthes J, Knopf-Marques H, Klüter H, Ghaemmaghami AM, Vrana NE, Kzhyshkowska J. Generation of anti-inflammatory macrophages for implants and regenerative medicine using self-standing release systems with a phenotype-fixing cytokine cocktail formulation. Acta Biomater. 2017 Apr 15;53:389-398.
- Stakheyeva M, Riabov V, Mitrofanova I, Litviakov N, Choynzonov E, Cherdyntseva N, Kzhyshkowska J. Role of the immune component of tumor microenvironment in the efficiency of cancer treatment: perspectives for the personalized therapy. Curr Pharm Des. 2017 Jul 14. doi: 10.2174/1381612823666170714161703. [Epub ahead of print]
- Mitrofanova I, Zavyalova M, Telegina N, Buldakov M, Riabov V, Cherdyntseva N, Kzhyshkowska J. Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology. 2017 Jan;222(1):101-109
- Moganti K, Li F, Schmuttermaier C, Riemann S, Klüter H, Gratchev A, Harmsen MC, Kzhyshkowska J J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology. 2016 Jul 22. pii: S0171-2985(16)30325-4. doi: 10.1016/j.imbio.2016.07.006. [Epub ahead of print]
- Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I, Gratchev A, Llopis Verdiell M, Sticht C, Schmuttermaier C, Schönhaber H, Weiss C, Fields AP, Simon-Keller K, Pfister F, Berlit S, Marx A, Arnold B, Goerdt S, Kzhyshkowska J. Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget. 2016 Apr 20.
- Alidori S, Bowman RL, Yarilin D, Romin Y, Barlas A, Mulvey JJ, Fujisawa S, Xu K, Ruggiero A, Riabov V, Thorek DL, Ulmert HD, Brea EJ, Behling K, Kzhyshkowska J, Manova-Todorova K, Scheinberg DA, McDevitt MR. Deconvoluting hepatic processing of carbon nanotubes. Nat Commun. 2016 Jul 29;7:12343.
- Kzhyshkowska J, Gudima A, Riabov V, Dollinger C, Lavalle P, Vrana NE. Macrophage responses to implants: prospects for personalized medicine. J Leukoc Biol. 2015 Dec;98(6):953-62.
- Buldakov M, Zavyalova M, Krakhmal N, Telegina N, Vtorushin S, Mitrofanova I, Riabov V, Yin S, Song B, Cherdyntseva N, Kzhyshkowska J. CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer. Immunobiology. 2015 Sep 8.
- Orekhov AN, Sobenin IA, Gavrilin MA, Gratchev A, Kotyashova SY, Nikiforov NG, Kzhyshkowska J. Macrophages in immunopathology of atherosclerosis: a target for diagnostics and therapy. Curr Pharm Des. 2015;21(9):1172-9.
- Nurgazieva D, Mickley A, Moganti K, Ming W, Ovsyi I, Popova A, Sachindra, Awad K, Wang N, Bieback K, Goerdt S, Kzhyshkowska J*, Gratchev A*. TGF-β1, but not bone morphogenetic proteins, activates Smad1/5 pathway in primary human macrophages and induces expression of proatherogenic genes. J Immunol. 2015 Jan 15;194(2):709-18. (*equal last authorship)
Kontextspalte
Leitung

Prof. (apl) Dr. rer. nat. Julia Kzhyshkowska
Telefon 0621383-9723
julia.kzhyshkowska@medma.uni-heidelberg.de
AG Members
Dr. Vladimir Riabov
Postdoc
Dr. Julia Michel
Postdoc
Marije Mossel
PhD Student
Tengfei Liu
PhD Student
Tatjana Sevastjanova
PhD Student
Michael Balduff
MD Student
Laura Matuschik
MD Student
Christina Schmuttermaier
Technical Assistant